

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM50365773 (CHEMBL1956552) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 260 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
School of Chemistry& Chemical Engineering of Guangxi Normal University Curated by ChEMBL | Assay Description Non-competitive inhibition of Electrophorus electricus AChE assessed as hydrolysis of acetylthiocholineiodide after 15 mins incubation by spectrophot... | Bioorg Med Chem Lett 22: 2257-61 (2012) Article DOI: 10.1016/j.bmcl.2012.01.090 BindingDB Entry DOI: 10.7270/Q2HH6KJX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Equus caballus (Horse)) | BDBM8961 (1,2,3,4-tetrahydro-9-acridinamine | 1,2,3,4-tetrah...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | n/a | n/a | 21 | n/a | n/a | n/a | n/a | n/a | n/a |
School of Chemistry& Chemical Engineering of Guangxi Normal University Curated by ChEMBL | Assay Description Inhibition of equine serum BChE after 15 mins incubation by spectrophotometry based Ellman's method | Bioorg Med Chem Lett 22: 2257-61 (2012) Article DOI: 10.1016/j.bmcl.2012.01.090 BindingDB Entry DOI: 10.7270/Q2HH6KJX | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM8961 (1,2,3,4-tetrahydro-9-acridinamine | 1,2,3,4-tetrah...) | UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | 130 | n/a | n/a | n/a | n/a | n/a | n/a |
School of Chemistry& Chemical Engineering of Guangxi Normal University Curated by ChEMBL | Assay Description Inhibition of Electrophorus electricus AChE using acetylthiocholine chloride as substrate after 15 mins incubation by Ellman's method | Bioorg Med Chem Lett 22: 2257-61 (2012) Article DOI: 10.1016/j.bmcl.2012.01.090 BindingDB Entry DOI: 10.7270/Q2HH6KJX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM50365773 (CHEMBL1956552) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 210 | n/a | n/a | n/a | n/a | n/a | n/a |
School of Chemistry& Chemical Engineering of Guangxi Normal University Curated by ChEMBL | Assay Description Inhibition of Electrophorus electricus AChE using acetylthiocholine chloride as substrate after 15 mins incubation by Ellman's method | Bioorg Med Chem Lett 22: 2257-61 (2012) Article DOI: 10.1016/j.bmcl.2012.01.090 BindingDB Entry DOI: 10.7270/Q2HH6KJX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM50365771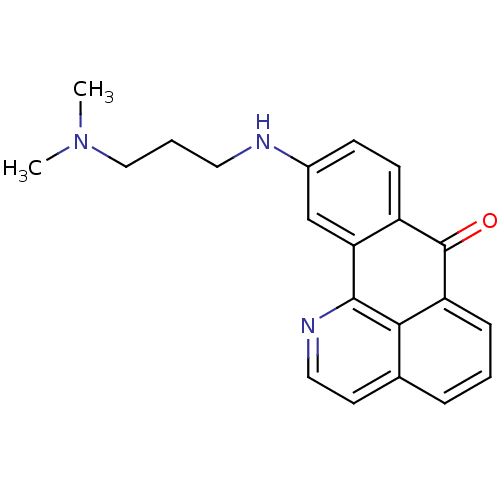 (CHEMBL1956550) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 490 | n/a | n/a | n/a | n/a | n/a | n/a |
School of Chemistry& Chemical Engineering of Guangxi Normal University Curated by ChEMBL | Assay Description Inhibition of Electrophorus electricus AChE using acetylthiocholine chloride as substrate after 15 mins incubation by Ellman's method | Bioorg Med Chem Lett 22: 2257-61 (2012) Article DOI: 10.1016/j.bmcl.2012.01.090 BindingDB Entry DOI: 10.7270/Q2HH6KJX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM50365781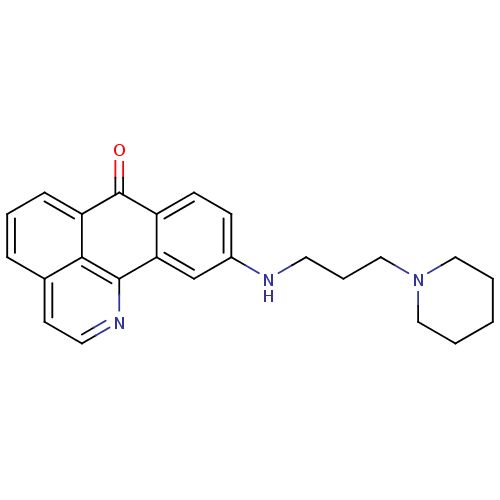 (CHEMBL1956560) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 580 | n/a | n/a | n/a | n/a | n/a | n/a |
School of Chemistry& Chemical Engineering of Guangxi Normal University Curated by ChEMBL | Assay Description Inhibition of Electrophorus electricus AChE using acetylthiocholine chloride as substrate after 15 mins incubation by Ellman's method | Bioorg Med Chem Lett 22: 2257-61 (2012) Article DOI: 10.1016/j.bmcl.2012.01.090 BindingDB Entry DOI: 10.7270/Q2HH6KJX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM50365772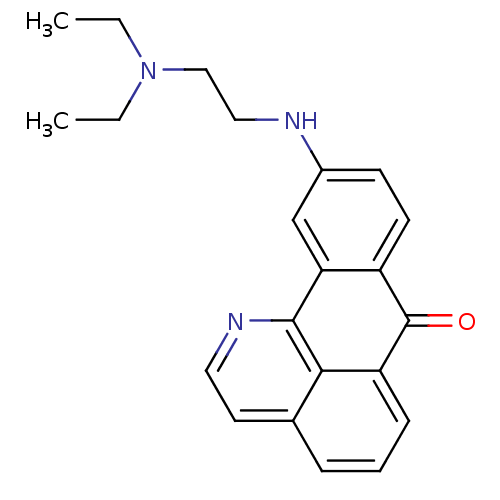 (CHEMBL1956551) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 720 | n/a | n/a | n/a | n/a | n/a | n/a |
School of Chemistry& Chemical Engineering of Guangxi Normal University Curated by ChEMBL | Assay Description Inhibition of Electrophorus electricus AChE using acetylthiocholine chloride as substrate after 15 mins incubation by Ellman's method | Bioorg Med Chem Lett 22: 2257-61 (2012) Article DOI: 10.1016/j.bmcl.2012.01.090 BindingDB Entry DOI: 10.7270/Q2HH6KJX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM50365779 (CHEMBL1956558) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 730 | n/a | n/a | n/a | n/a | n/a | n/a |
School of Chemistry& Chemical Engineering of Guangxi Normal University Curated by ChEMBL | Assay Description Inhibition of Electrophorus electricus AChE using acetylthiocholine chloride as substrate after 15 mins incubation by Ellman's method | Bioorg Med Chem Lett 22: 2257-61 (2012) Article DOI: 10.1016/j.bmcl.2012.01.090 BindingDB Entry DOI: 10.7270/Q2HH6KJX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM50365780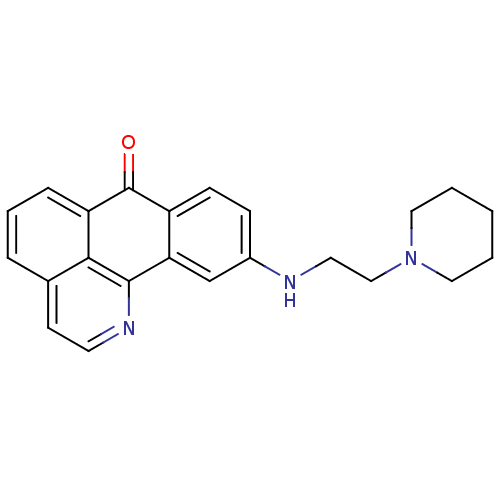 (CHEMBL1956559) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.10E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
School of Chemistry& Chemical Engineering of Guangxi Normal University Curated by ChEMBL | Assay Description Inhibition of Electrophorus electricus AChE using acetylthiocholine chloride as substrate after 15 mins incubation by Ellman's method | Bioorg Med Chem Lett 22: 2257-61 (2012) Article DOI: 10.1016/j.bmcl.2012.01.090 BindingDB Entry DOI: 10.7270/Q2HH6KJX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Equus caballus (Horse)) | BDBM50365772 (CHEMBL1956551) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.13E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
School of Chemistry& Chemical Engineering of Guangxi Normal University Curated by ChEMBL | Assay Description Inhibition of equine serum BChE after 15 mins incubation by spectrophotometry based Ellman's method | Bioorg Med Chem Lett 22: 2257-61 (2012) Article DOI: 10.1016/j.bmcl.2012.01.090 BindingDB Entry DOI: 10.7270/Q2HH6KJX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Equus caballus (Horse)) | BDBM50365771 (CHEMBL1956550) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.19E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
School of Chemistry& Chemical Engineering of Guangxi Normal University Curated by ChEMBL | Assay Description Inhibition of equine serum BChE after 15 mins incubation by spectrophotometry based Ellman's method | Bioorg Med Chem Lett 22: 2257-61 (2012) Article DOI: 10.1016/j.bmcl.2012.01.090 BindingDB Entry DOI: 10.7270/Q2HH6KJX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Equus caballus (Horse)) | BDBM50365780 (CHEMBL1956559) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.42E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
School of Chemistry& Chemical Engineering of Guangxi Normal University Curated by ChEMBL | Assay Description Inhibition of equine serum BChE after 15 mins incubation by spectrophotometry based Ellman's method | Bioorg Med Chem Lett 22: 2257-61 (2012) Article DOI: 10.1016/j.bmcl.2012.01.090 BindingDB Entry DOI: 10.7270/Q2HH6KJX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Equus caballus (Horse)) | BDBM50365781 (CHEMBL1956560) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.54E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
School of Chemistry& Chemical Engineering of Guangxi Normal University Curated by ChEMBL | Assay Description Inhibition of equine serum BChE after 15 mins incubation by spectrophotometry based Ellman's method | Bioorg Med Chem Lett 22: 2257-61 (2012) Article DOI: 10.1016/j.bmcl.2012.01.090 BindingDB Entry DOI: 10.7270/Q2HH6KJX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Equus caballus (Horse)) | BDBM50365770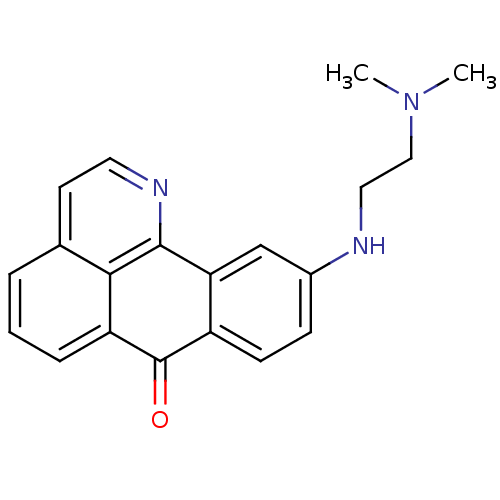 (CHEMBL1956549) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.89E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
School of Chemistry& Chemical Engineering of Guangxi Normal University Curated by ChEMBL | Assay Description Inhibition of equine serum BChE after 15 mins incubation by spectrophotometry based Ellman's method | Bioorg Med Chem Lett 22: 2257-61 (2012) Article DOI: 10.1016/j.bmcl.2012.01.090 BindingDB Entry DOI: 10.7270/Q2HH6KJX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM50365778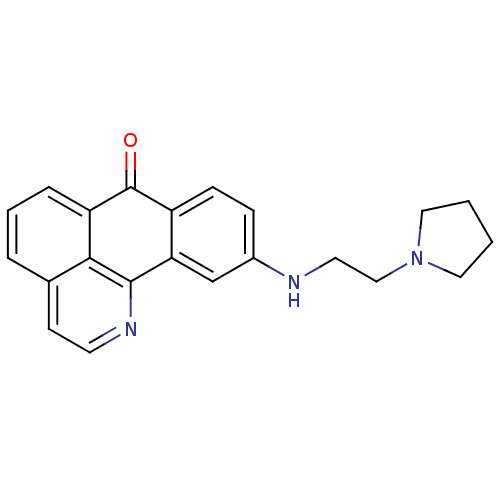 (CHEMBL1956557) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
School of Chemistry& Chemical Engineering of Guangxi Normal University Curated by ChEMBL | Assay Description Inhibition of Electrophorus electricus AChE using acetylthiocholine chloride as substrate after 15 mins incubation by Ellman's method | Bioorg Med Chem Lett 22: 2257-61 (2012) Article DOI: 10.1016/j.bmcl.2012.01.090 BindingDB Entry DOI: 10.7270/Q2HH6KJX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM50365770 (CHEMBL1956549) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2.03E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
School of Chemistry& Chemical Engineering of Guangxi Normal University Curated by ChEMBL | Assay Description Inhibition of Electrophorus electricus AChE using acetylthiocholine chloride as substrate after 15 mins incubation by Ellman's method | Bioorg Med Chem Lett 22: 2257-61 (2012) Article DOI: 10.1016/j.bmcl.2012.01.090 BindingDB Entry DOI: 10.7270/Q2HH6KJX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM50365775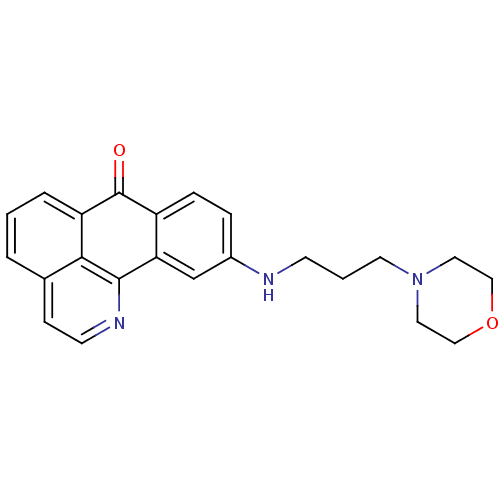 (CHEMBL1956554) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2.20E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
School of Chemistry& Chemical Engineering of Guangxi Normal University Curated by ChEMBL | Assay Description Inhibition of Electrophorus electricus AChE using acetylthiocholine chloride as substrate after 15 mins incubation by Ellman's method | Bioorg Med Chem Lett 22: 2257-61 (2012) Article DOI: 10.1016/j.bmcl.2012.01.090 BindingDB Entry DOI: 10.7270/Q2HH6KJX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Equus caballus (Horse)) | BDBM50365778 (CHEMBL1956557) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2.78E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
School of Chemistry& Chemical Engineering of Guangxi Normal University Curated by ChEMBL | Assay Description Inhibition of equine serum BChE after 15 mins incubation by spectrophotometry based Ellman's method | Bioorg Med Chem Lett 22: 2257-61 (2012) Article DOI: 10.1016/j.bmcl.2012.01.090 BindingDB Entry DOI: 10.7270/Q2HH6KJX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Equus caballus (Horse)) | BDBM50365773 (CHEMBL1956552) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2.81E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
School of Chemistry& Chemical Engineering of Guangxi Normal University Curated by ChEMBL | Assay Description Inhibition of equine serum BChE after 15 mins incubation by spectrophotometry based Ellman's method | Bioorg Med Chem Lett 22: 2257-61 (2012) Article DOI: 10.1016/j.bmcl.2012.01.090 BindingDB Entry DOI: 10.7270/Q2HH6KJX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Equus caballus (Horse)) | BDBM50365779 (CHEMBL1956558) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 4.97E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
School of Chemistry& Chemical Engineering of Guangxi Normal University Curated by ChEMBL | Assay Description Inhibition of equine serum BChE after 15 mins incubation by spectrophotometry based Ellman's method | Bioorg Med Chem Lett 22: 2257-61 (2012) Article DOI: 10.1016/j.bmcl.2012.01.090 BindingDB Entry DOI: 10.7270/Q2HH6KJX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM50365774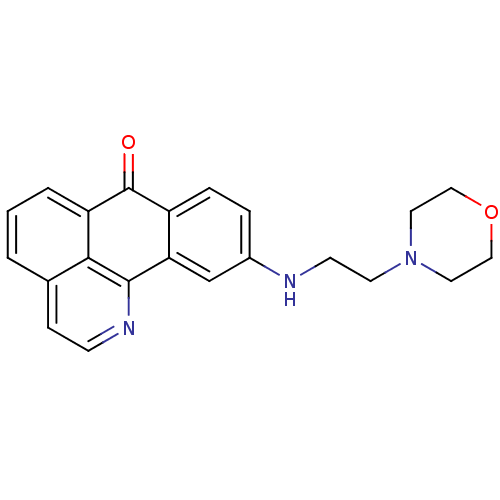 (CHEMBL1956553) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 5.76E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
School of Chemistry& Chemical Engineering of Guangxi Normal University Curated by ChEMBL | Assay Description Inhibition of Electrophorus electricus AChE using acetylthiocholine chloride as substrate after 15 mins incubation by Ellman's method | Bioorg Med Chem Lett 22: 2257-61 (2012) Article DOI: 10.1016/j.bmcl.2012.01.090 BindingDB Entry DOI: 10.7270/Q2HH6KJX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Equus caballus (Horse)) | BDBM50365775 (CHEMBL1956554) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 6.94E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
School of Chemistry& Chemical Engineering of Guangxi Normal University Curated by ChEMBL | Assay Description Inhibition of equine serum BChE after 15 mins incubation by spectrophotometry based Ellman's method | Bioorg Med Chem Lett 22: 2257-61 (2012) Article DOI: 10.1016/j.bmcl.2012.01.090 BindingDB Entry DOI: 10.7270/Q2HH6KJX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM50365776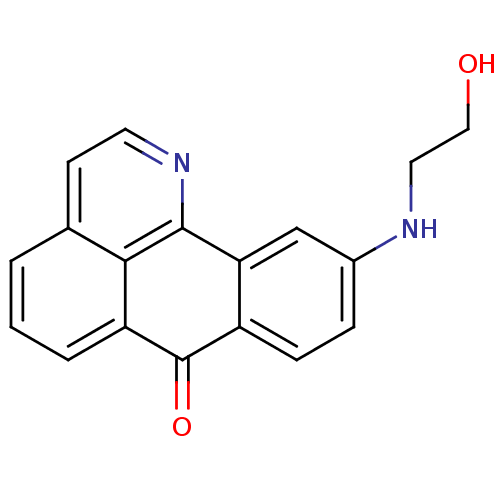 (CHEMBL1956555) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.17E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
School of Chemistry& Chemical Engineering of Guangxi Normal University Curated by ChEMBL | Assay Description Inhibition of Electrophorus electricus AChE using acetylthiocholine chloride as substrate after 15 mins incubation by Ellman's method | Bioorg Med Chem Lett 22: 2257-61 (2012) Article DOI: 10.1016/j.bmcl.2012.01.090 BindingDB Entry DOI: 10.7270/Q2HH6KJX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Equus caballus (Horse)) | BDBM50365774 (CHEMBL1956553) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2.59E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
School of Chemistry& Chemical Engineering of Guangxi Normal University Curated by ChEMBL | Assay Description Inhibition of equine serum BChE after 15 mins incubation by spectrophotometry based Ellman's method | Bioorg Med Chem Lett 22: 2257-61 (2012) Article DOI: 10.1016/j.bmcl.2012.01.090 BindingDB Entry DOI: 10.7270/Q2HH6KJX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM50365777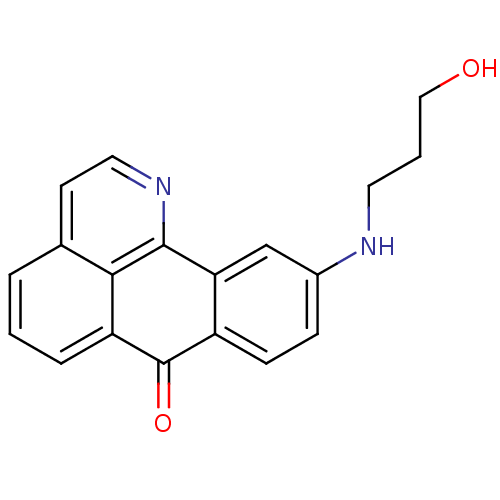 (CHEMBL1956556) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 5.58E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
School of Chemistry& Chemical Engineering of Guangxi Normal University Curated by ChEMBL | Assay Description Inhibition of Electrophorus electricus AChE using acetylthiocholine chloride as substrate after 15 mins incubation by Ellman's method | Bioorg Med Chem Lett 22: 2257-61 (2012) Article DOI: 10.1016/j.bmcl.2012.01.090 BindingDB Entry DOI: 10.7270/Q2HH6KJX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM50365769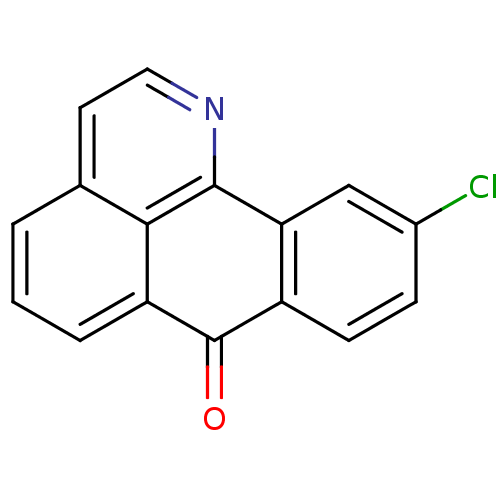 (CHEMBL1956548) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
School of Chemistry& Chemical Engineering of Guangxi Normal University Curated by ChEMBL | Assay Description Inhibition of Electrophorus electricus AChE using acetylthiocholine chloride as substrate after 15 mins incubation by Ellman's method | Bioorg Med Chem Lett 22: 2257-61 (2012) Article DOI: 10.1016/j.bmcl.2012.01.090 BindingDB Entry DOI: 10.7270/Q2HH6KJX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Equus caballus (Horse)) | BDBM50365769 (CHEMBL1956548) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
School of Chemistry& Chemical Engineering of Guangxi Normal University Curated by ChEMBL | Assay Description Inhibition of equine serum BChE after 15 mins incubation by spectrophotometry based Ellman's method | Bioorg Med Chem Lett 22: 2257-61 (2012) Article DOI: 10.1016/j.bmcl.2012.01.090 BindingDB Entry DOI: 10.7270/Q2HH6KJX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Equus caballus (Horse)) | BDBM50365776 (CHEMBL1956555) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
School of Chemistry& Chemical Engineering of Guangxi Normal University Curated by ChEMBL | Assay Description Inhibition of equine serum BChE after 15 mins incubation by spectrophotometry based Ellman's method | Bioorg Med Chem Lett 22: 2257-61 (2012) Article DOI: 10.1016/j.bmcl.2012.01.090 BindingDB Entry DOI: 10.7270/Q2HH6KJX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Equus caballus (Horse)) | BDBM50365777 (CHEMBL1956556) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
School of Chemistry& Chemical Engineering of Guangxi Normal University Curated by ChEMBL | Assay Description Inhibition of equine serum BChE after 15 mins incubation by spectrophotometry based Ellman's method | Bioorg Med Chem Lett 22: 2257-61 (2012) Article DOI: 10.1016/j.bmcl.2012.01.090 BindingDB Entry DOI: 10.7270/Q2HH6KJX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||