 Found 32 hits Enz. Inhib. hit(s) with all data for entry = 50049176
Found 32 hits Enz. Inhib. hit(s) with all data for entry = 50049176 Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Dihydrofolate reductase
(Homo sapiens (Human)) | BDBM66082

((2S)-2-[[4-[(2,4-diaminopteridin-6-yl)methyl-methy...)Show SMILES CN(Cc1cnc2nc(N)nc(N)c2n1)c1ccc(cc1)C(=O)N[C@@H](CCC(O)=O)C(O)=O Show InChI InChI=1S/C20H22N8O5/c1-28(9-11-8-23-17-15(24-11)16(21)26-20(22)27-17)12-4-2-10(3-5-12)18(31)25-13(19(32)33)6-7-14(29)30/h2-5,8,13H,6-7,9H2,1H3,(H,25,31)(H,29,30)(H,32,33)(H4,21,22,23,26,27)/t13-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
DrugBank
MCE
KEGG
PC cid
PC sid
PDB
UniChem
Similars
| DrugBank
PDB
Article
PubMed
| n/a | n/a | 22 | n/a | n/a | n/a | n/a | n/a | n/a |
Duquesne University
Curated by ChEMBL
| Assay Description
Inhibition of rat hippocampal Gamma-aminobutyric acid type B receptor (weak agonist) |
Bioorg Med Chem Lett 27: 1602-1607 (2017)
Article DOI: 10.1016/j.bmcl.2017.02.018
BindingDB Entry DOI: 10.7270/Q2P55QR0 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Polyunsaturated fatty acid lipoxygenase ALOX15B
(Rattus norvegicus) | BDBM50235696

(CHEMBL4060383)Show SMILES Nc1nc(N)c2c(n1)[nH]c1cccc(Sc3ccc4ccccc4c3)c21 Show InChI InChI=1S/C20H15N5S/c21-18-17-16-14(23-19(17)25-20(22)24-18)6-3-7-15(16)26-13-9-8-11-4-1-2-5-12(11)10-13/h1-10H,(H5,21,22,23,24,25) | KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 120 | n/a | n/a | n/a | n/a | n/a | n/a |
Duquesne University
Curated by ChEMBL
| Assay Description
Inhibition of human thymidylate synthase using dUMP/(6R,S)-tetrahydrofolate as substrate/co-factor by spectrophotometric method |
Bioorg Med Chem Lett 27: 1602-1607 (2017)
Article DOI: 10.1016/j.bmcl.2017.02.018
BindingDB Entry DOI: 10.7270/Q2P55QR0 |
More data for this
Ligand-Target Pair | |
Polyunsaturated fatty acid lipoxygenase ALOX15B
(Rattus norvegicus) | BDBM50235700
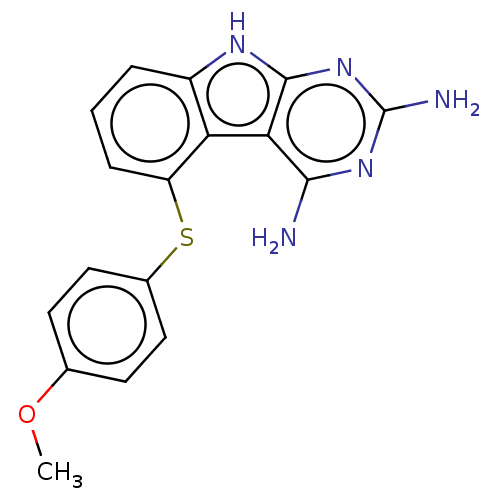
(CHEMBL4096329)Show InChI InChI=1S/C17H15N5OS/c1-23-9-5-7-10(8-6-9)24-12-4-2-3-11-13(12)14-15(18)21-17(19)22-16(14)20-11/h2-8H,1H3,(H5,18,19,20,21,22) | KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 140 | n/a | n/a | n/a | n/a | n/a | n/a |
Duquesne University
Curated by ChEMBL
| Assay Description
Inhibition of human thymidylate synthase using dUMP/(6R,S)-tetrahydrofolate as substrate/co-factor by spectrophotometric method |
Bioorg Med Chem Lett 27: 1602-1607 (2017)
Article DOI: 10.1016/j.bmcl.2017.02.018
BindingDB Entry DOI: 10.7270/Q2P55QR0 |
More data for this
Ligand-Target Pair | |
Epidermal growth factor receptor
(Homo sapiens (Human)) | BDBM50235695
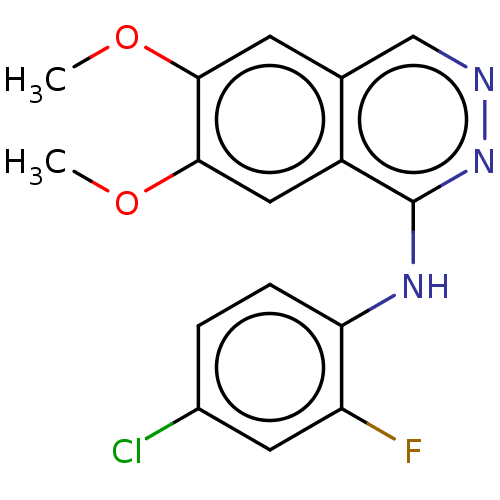
(CHEMBL4087709)Show InChI InChI=1S/C16H13ClFN3O2/c1-22-14-5-9-8-19-21-16(11(9)7-15(14)23-2)20-13-4-3-10(17)6-12(13)18/h3-8H,1-2H3,(H,20,21) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 200 | n/a | n/a | n/a | n/a | n/a | n/a |
Duquesne University
Curated by ChEMBL
| Assay Description
Binding affinity against Gamma-aminobutyric acid type B receptor in rat brain synaptosomes after 30 minutes |
Bioorg Med Chem Lett 27: 1602-1607 (2017)
Article DOI: 10.1016/j.bmcl.2017.02.018
BindingDB Entry DOI: 10.7270/Q2P55QR0 |
More data for this
Ligand-Target Pair | |
Polyunsaturated fatty acid lipoxygenase ALOX15B
(Rattus norvegicus) | BDBM50027655

(CHEBI:5847 | Raltitrexed | Tomudex | US9422297, Ra...)Show SMILES CN(Cc1ccc2[nH]c(C)nc(=O)c2c1)c1ccc(s1)C(=O)N[C@@H](CCC(O)=O)C(O)=O |r| Show InChI InChI=1S/C21H22N4O6S/c1-11-22-14-4-3-12(9-13(14)19(28)23-11)10-25(2)17-7-6-16(32-17)20(29)24-15(21(30)31)5-8-18(26)27/h3-4,6-7,9,15H,5,8,10H2,1-2H3,(H,24,29)(H,26,27)(H,30,31)(H,22,23,28)/t15-/m0/s1 | KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
DrugBank
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 380 | n/a | n/a | n/a | n/a | n/a | n/a |
Duquesne University
Curated by ChEMBL
| Assay Description
Inhibition of human thymidylate synthase using dUMP/(6R,S)-tetrahydrofolate as substrate/co-factor by spectrophotometric method |
Bioorg Med Chem Lett 27: 1602-1607 (2017)
Article DOI: 10.1016/j.bmcl.2017.02.018
BindingDB Entry DOI: 10.7270/Q2P55QR0 |
More data for this
Ligand-Target Pair | |
Polyunsaturated fatty acid lipoxygenase ALOX15B
(Rattus norvegicus) | BDBM50235699
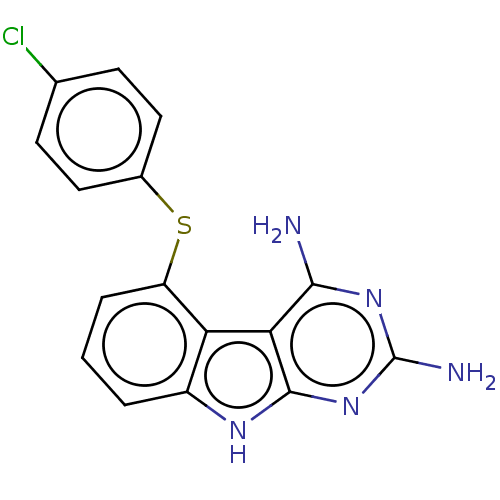
(CHEMBL4079829)Show SMILES Nc1nc(N)c2c(n1)[nH]c1cccc(Sc3ccc(Cl)cc3)c21 Show InChI InChI=1S/C16H12ClN5S/c17-8-4-6-9(7-5-8)23-11-3-1-2-10-12(11)13-14(18)21-16(19)22-15(13)20-10/h1-7H,(H5,18,19,20,21,22) | KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 480 | n/a | n/a | n/a | n/a | n/a | n/a |
Duquesne University
Curated by ChEMBL
| Assay Description
Inhibition of human thymidylate synthase using dUMP/(6R,S)-tetrahydrofolate as substrate/co-factor by spectrophotometric method |
Bioorg Med Chem Lett 27: 1602-1607 (2017)
Article DOI: 10.1016/j.bmcl.2017.02.018
BindingDB Entry DOI: 10.7270/Q2P55QR0 |
More data for this
Ligand-Target Pair | |
Polyunsaturated fatty acid lipoxygenase ALOX15B
(Rattus norvegicus) | BDBM50235698
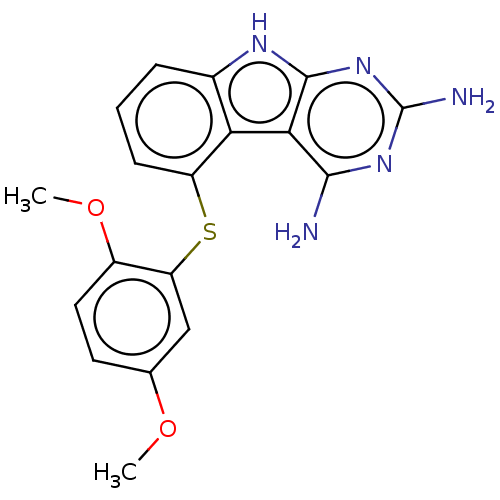
(CHEMBL4069070)Show SMILES COc1ccc(OC)c(Sc2cccc3[nH]c4nc(N)nc(N)c4c23)c1 Show InChI InChI=1S/C18H17N5O2S/c1-24-9-6-7-11(25-2)13(8-9)26-12-5-3-4-10-14(12)15-16(19)22-18(20)23-17(15)21-10/h3-8H,1-2H3,(H5,19,20,21,22,23) | KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 1.10E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Duquesne University
Curated by ChEMBL
| Assay Description
Inhibition of human thymidylate synthase using dUMP/(6R,S)-tetrahydrofolate as substrate/co-factor by spectrophotometric method |
Bioorg Med Chem Lett 27: 1602-1607 (2017)
Article DOI: 10.1016/j.bmcl.2017.02.018
BindingDB Entry DOI: 10.7270/Q2P55QR0 |
More data for this
Ligand-Target Pair | |
Polyunsaturated fatty acid lipoxygenase ALOX15B
(Rattus norvegicus) | BDBM50235697
(CHEMBL4088617)Show SMILES Nc1nc(N)c2c(n1)[nH]c1cccc(Sc3cccc4ccccc34)c21 Show InChI InChI=1S/C20H15N5S/c21-18-17-16-13(23-19(17)25-20(22)24-18)8-4-10-15(16)26-14-9-3-6-11-5-1-2-7-12(11)14/h1-10H,(H5,21,22,23,24,25) | KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 2.30E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Duquesne University
Curated by ChEMBL
| Assay Description
Inhibition of human thymidylate synthase using dUMP/(6R,S)-tetrahydrofolate as substrate/co-factor by spectrophotometric method |
Bioorg Med Chem Lett 27: 1602-1607 (2017)
Article DOI: 10.1016/j.bmcl.2017.02.018
BindingDB Entry DOI: 10.7270/Q2P55QR0 |
More data for this
Ligand-Target Pair | |
Platelet-derived growth factor receptor beta
(Homo sapiens (Human)) | BDBM17747

((3Z)-3-{[4-(dimethylamino)phenyl]methylidene}-2,3-...)Show InChI InChI=1S/C17H16N2O/c1-19(2)13-9-7-12(8-10-13)11-15-14-5-3-4-6-16(14)18-17(15)20/h3-11H,1-2H3,(H,18,20)/b15-11- | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 3.70E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Duquesne University
Curated by ChEMBL
| Assay Description
Inhibition of PDGF-BB-induced PDGFR-beta phosphorylation in human SF-539 cells preincubated for 60 mins followed by PDGF-BB induction measured after ... |
Bioorg Med Chem Lett 27: 1602-1607 (2017)
Article DOI: 10.1016/j.bmcl.2017.02.018
BindingDB Entry DOI: 10.7270/Q2P55QR0 |
More data for this
Ligand-Target Pair | |
Epidermal growth factor receptor
(Homo sapiens (Human)) | BDBM50235698

(CHEMBL4069070)Show SMILES COc1ccc(OC)c(Sc2cccc3[nH]c4nc(N)nc(N)c4c23)c1 Show InChI InChI=1S/C18H17N5O2S/c1-24-9-6-7-11(25-2)13(8-9)26-12-5-3-4-10-14(12)15-16(19)22-18(20)23-17(15)21-10/h3-8H,1-2H3,(H5,19,20,21,22,23) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 4.30E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Duquesne University
Curated by ChEMBL
| Assay Description
Inhibition of EGF-induced EGFR phosphorylation in human A431 cells preincubated for 60 mins followed by EGF induction measured after 10 mins by ELISA |
Bioorg Med Chem Lett 27: 1602-1607 (2017)
Article DOI: 10.1016/j.bmcl.2017.02.018
BindingDB Entry DOI: 10.7270/Q2P55QR0 |
More data for this
Ligand-Target Pair | |
Epidermal growth factor receptor
(Homo sapiens (Human)) | BDBM50235699

(CHEMBL4079829)Show SMILES Nc1nc(N)c2c(n1)[nH]c1cccc(Sc3ccc(Cl)cc3)c21 Show InChI InChI=1S/C16H12ClN5S/c17-8-4-6-9(7-5-8)23-11-3-1-2-10-12(11)13-14(18)21-16(19)22-15(13)20-10/h1-7H,(H5,18,19,20,21,22) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 8.30E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Duquesne University
Curated by ChEMBL
| Assay Description
Inhibition of EGF-induced EGFR phosphorylation in human A431 cells preincubated for 60 mins followed by EGF induction measured after 10 mins by ELISA |
Bioorg Med Chem Lett 27: 1602-1607 (2017)
Article DOI: 10.1016/j.bmcl.2017.02.018
BindingDB Entry DOI: 10.7270/Q2P55QR0 |
More data for this
Ligand-Target Pair | |
Polyunsaturated fatty acid lipoxygenase ALOX15B
(Rattus norvegicus) | BDBM50027656
(CHEBI:63616 | LY-2315 | LY-231514 | PEMETREXED | U...)Show SMILES Nc1nc2[nH]cc(CCc3ccc(cc3)C(=O)N[C@@H](CCC(O)=O)C(O)=O)c2c(=O)[nH]1 Show InChI InChI=1S/C20H21N5O6/c21-20-24-16-15(18(29)25-20)12(9-22-16)6-3-10-1-4-11(5-2-10)17(28)23-13(19(30)31)7-8-14(26)27/h1-2,4-5,9,13H,3,6-8H2,(H,23,28)(H,26,27)(H,30,31)(H4,21,22,24,25,29)/t13-/m0/s1 | KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 9.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Duquesne University
Curated by ChEMBL
| Assay Description
Inhibition of human thymidylate synthase using dUMP/(6R,S)-tetrahydrofolate as substrate/co-factor by spectrophotometric method |
Bioorg Med Chem Lett 27: 1602-1607 (2017)
Article DOI: 10.1016/j.bmcl.2017.02.018
BindingDB Entry DOI: 10.7270/Q2P55QR0 |
More data for this
Ligand-Target Pair | |
Vascular endothelial growth factor receptor 2
(Homo sapiens (Human)) | BDBM50235698

(CHEMBL4069070)Show SMILES COc1ccc(OC)c(Sc2cccc3[nH]c4nc(N)nc(N)c4c23)c1 Show InChI InChI=1S/C18H17N5O2S/c1-24-9-6-7-11(25-2)13(8-9)26-12-5-3-4-10-14(12)15-16(19)22-18(20)23-17(15)21-10/h3-8H,1-2H3,(H5,19,20,21,22,23) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 1.02E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Duquesne University
Curated by ChEMBL
| Assay Description
Inhibition of VEGF-induced VEGFR2 phosphorylation in human U251 cells preincubated for 60 mins followed by VEGF induction measured after 10 mins by E... |
Bioorg Med Chem Lett 27: 1602-1607 (2017)
Article DOI: 10.1016/j.bmcl.2017.02.018
BindingDB Entry DOI: 10.7270/Q2P55QR0 |
More data for this
Ligand-Target Pair | |
Vascular endothelial growth factor receptor 2
(Homo sapiens (Human)) | BDBM4810
((3Z)-3-[(3,5-dimethyl-1H-pyrrol-2-yl)methylidene]-...)Show InChI InChI=1S/C15H14N2O/c1-9-7-10(2)16-14(9)8-12-11-5-3-4-6-13(11)17-15(12)18/h3-8,16H,1-2H3,(H,17,18)/b12-8- | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 1.20E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Duquesne University
Curated by ChEMBL
| Assay Description
Inhibition of VEGF-induced VEGFR2 phosphorylation in human U251 cells preincubated for 60 mins followed by VEGF induction measured after 10 mins by E... |
Bioorg Med Chem Lett 27: 1602-1607 (2017)
Article DOI: 10.1016/j.bmcl.2017.02.018
BindingDB Entry DOI: 10.7270/Q2P55QR0 |
More data for this
Ligand-Target Pair | |
Epidermal growth factor receptor
(Homo sapiens (Human)) | BDBM50235696

(CHEMBL4060383)Show SMILES Nc1nc(N)c2c(n1)[nH]c1cccc(Sc3ccc4ccccc4c3)c21 Show InChI InChI=1S/C20H15N5S/c21-18-17-16-14(23-19(17)25-20(22)24-18)6-3-7-15(16)26-13-9-8-11-4-1-2-5-12(11)10-13/h1-10H,(H5,21,22,23,24,25) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Duquesne University
Curated by ChEMBL
| Assay Description
Inhibition of EGF-induced EGFR phosphorylation in human A431 cells preincubated for 60 mins followed by EGF induction measured after 10 mins by ELISA |
Bioorg Med Chem Lett 27: 1602-1607 (2017)
Article DOI: 10.1016/j.bmcl.2017.02.018
BindingDB Entry DOI: 10.7270/Q2P55QR0 |
More data for this
Ligand-Target Pair | |
Epidermal growth factor receptor
(Homo sapiens (Human)) | BDBM50235700

(CHEMBL4096329)Show InChI InChI=1S/C17H15N5OS/c1-23-9-5-7-10(8-6-9)24-12-4-2-3-11-13(12)14-15(18)21-17(19)22-16(14)20-11/h2-8H,1H3,(H5,18,19,20,21,22) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 2.08E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Duquesne University
Curated by ChEMBL
| Assay Description
Inhibition of EGF-induced EGFR phosphorylation in human A431 cells preincubated for 60 mins followed by EGF induction measured after 10 mins by ELISA |
Bioorg Med Chem Lett 27: 1602-1607 (2017)
Article DOI: 10.1016/j.bmcl.2017.02.018
BindingDB Entry DOI: 10.7270/Q2P55QR0 |
More data for this
Ligand-Target Pair | |
Dihydrofolate reductase
(Homo sapiens (Human)) | BDBM50235698

(CHEMBL4069070)Show SMILES COc1ccc(OC)c(Sc2cccc3[nH]c4nc(N)nc(N)c4c23)c1 Show InChI InChI=1S/C18H17N5O2S/c1-24-9-6-7-11(25-2)13(8-9)26-12-5-3-4-10-14(12)15-16(19)22-18(20)23-17(15)21-10/h3-8H,1-2H3,(H5,19,20,21,22,23) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | >2.70E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Duquesne University
Curated by ChEMBL
| Assay Description
Inhibition of human DHFR using dihydrofolate as substrate by spectrophotometric method |
Bioorg Med Chem Lett 27: 1602-1607 (2017)
Article DOI: 10.1016/j.bmcl.2017.02.018
BindingDB Entry DOI: 10.7270/Q2P55QR0 |
More data for this
Ligand-Target Pair | |
Dihydrofolate reductase
(Homo sapiens (Human)) | BDBM50235697

(CHEMBL4088617)Show SMILES Nc1nc(N)c2c(n1)[nH]c1cccc(Sc3cccc4ccccc34)c21 Show InChI InChI=1S/C20H15N5S/c21-18-17-16-13(23-19(17)25-20(22)24-18)8-4-10-15(16)26-14-9-3-6-11-5-1-2-7-12(11)14/h1-10H,(H5,21,22,23,24,25) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | >2.70E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Duquesne University
Curated by ChEMBL
| Assay Description
Inhibition of human DHFR using dihydrofolate as substrate by spectrophotometric method |
Bioorg Med Chem Lett 27: 1602-1607 (2017)
Article DOI: 10.1016/j.bmcl.2017.02.018
BindingDB Entry DOI: 10.7270/Q2P55QR0 |
More data for this
Ligand-Target Pair | |
Dihydrofolate reductase
(Homo sapiens (Human)) | BDBM50235696

(CHEMBL4060383)Show SMILES Nc1nc(N)c2c(n1)[nH]c1cccc(Sc3ccc4ccccc4c3)c21 Show InChI InChI=1S/C20H15N5S/c21-18-17-16-14(23-19(17)25-20(22)24-18)6-3-7-15(16)26-13-9-8-11-4-1-2-5-12(11)10-13/h1-10H,(H5,21,22,23,24,25) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | >2.80E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Duquesne University
Curated by ChEMBL
| Assay Description
Inhibition of human DHFR using dihydrofolate as substrate by spectrophotometric method |
Bioorg Med Chem Lett 27: 1602-1607 (2017)
Article DOI: 10.1016/j.bmcl.2017.02.018
BindingDB Entry DOI: 10.7270/Q2P55QR0 |
More data for this
Ligand-Target Pair | |
Dihydrofolate reductase
(Homo sapiens (Human)) | BDBM50235699

(CHEMBL4079829)Show SMILES Nc1nc(N)c2c(n1)[nH]c1cccc(Sc3ccc(Cl)cc3)c21 Show InChI InChI=1S/C16H12ClN5S/c17-8-4-6-9(7-5-8)23-11-3-1-2-10-12(11)13-14(18)21-16(19)22-15(13)20-10/h1-7H,(H5,18,19,20,21,22) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | >2.90E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Duquesne University
Curated by ChEMBL
| Assay Description
Inhibition of mammalian Gamma-aminobutyric acid type B receptor (potent agonist) |
Bioorg Med Chem Lett 27: 1602-1607 (2017)
Article DOI: 10.1016/j.bmcl.2017.02.018
BindingDB Entry DOI: 10.7270/Q2P55QR0 |
More data for this
Ligand-Target Pair | |
Dihydrofolate reductase
(Homo sapiens (Human)) | BDBM50235700

(CHEMBL4096329)Show InChI InChI=1S/C17H15N5OS/c1-23-9-5-7-10(8-6-9)24-12-4-2-3-11-13(12)14-15(18)21-17(19)22-16(14)20-11/h2-8H,1H3,(H5,18,19,20,21,22) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | >3.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Duquesne University
Curated by ChEMBL
| Assay Description
Inhibition of human DHFR using dihydrofolate as substrate by spectrophotometric method |
Bioorg Med Chem Lett 27: 1602-1607 (2017)
Article DOI: 10.1016/j.bmcl.2017.02.018
BindingDB Entry DOI: 10.7270/Q2P55QR0 |
More data for this
Ligand-Target Pair | |
Platelet-derived growth factor receptor beta
(Homo sapiens (Human)) | BDBM50235697

(CHEMBL4088617)Show SMILES Nc1nc(N)c2c(n1)[nH]c1cccc(Sc3cccc4ccccc34)c21 Show InChI InChI=1S/C20H15N5S/c21-18-17-16-13(23-19(17)25-20(22)24-18)8-4-10-15(16)26-14-9-3-6-11-5-1-2-7-12(11)14/h1-10H,(H5,21,22,23,24,25) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 5.82E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Duquesne University
Curated by ChEMBL
| Assay Description
Inhibition of PDGF-BB-induced PDGFR-beta phosphorylation in human SF-539 cells preincubated for 60 mins followed by PDGF-BB induction measured after ... |
Bioorg Med Chem Lett 27: 1602-1607 (2017)
Article DOI: 10.1016/j.bmcl.2017.02.018
BindingDB Entry DOI: 10.7270/Q2P55QR0 |
More data for this
Ligand-Target Pair | |
Platelet-derived growth factor receptor beta
(Homo sapiens (Human)) | BDBM50235700

(CHEMBL4096329)Show InChI InChI=1S/C17H15N5OS/c1-23-9-5-7-10(8-6-9)24-12-4-2-3-11-13(12)14-15(18)21-17(19)22-16(14)20-11/h2-8H,1H3,(H5,18,19,20,21,22) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 6.05E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Duquesne University
Curated by ChEMBL
| Assay Description
Inhibition of PDGF-BB-induced PDGFR-beta phosphorylation in human SF-539 cells preincubated for 60 mins followed by PDGF-BB induction measured after ... |
Bioorg Med Chem Lett 27: 1602-1607 (2017)
Article DOI: 10.1016/j.bmcl.2017.02.018
BindingDB Entry DOI: 10.7270/Q2P55QR0 |
More data for this
Ligand-Target Pair | |
Platelet-derived growth factor receptor beta
(Homo sapiens (Human)) | BDBM50235696

(CHEMBL4060383)Show SMILES Nc1nc(N)c2c(n1)[nH]c1cccc(Sc3ccc4ccccc4c3)c21 Show InChI InChI=1S/C20H15N5S/c21-18-17-16-14(23-19(17)25-20(22)24-18)6-3-7-15(16)26-13-9-8-11-4-1-2-5-12(11)10-13/h1-10H,(H5,21,22,23,24,25) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 9.01E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Duquesne University
Curated by ChEMBL
| Assay Description
Inhibition of PDGF-BB-induced PDGFR-beta phosphorylation in human SF-539 cells preincubated for 60 mins followed by PDGF-BB induction measured after ... |
Bioorg Med Chem Lett 27: 1602-1607 (2017)
Article DOI: 10.1016/j.bmcl.2017.02.018
BindingDB Entry DOI: 10.7270/Q2P55QR0 |
More data for this
Ligand-Target Pair | |
Vascular endothelial growth factor receptor 2
(Homo sapiens (Human)) | BDBM50235696

(CHEMBL4060383)Show SMILES Nc1nc(N)c2c(n1)[nH]c1cccc(Sc3ccc4ccccc4c3)c21 Show InChI InChI=1S/C20H15N5S/c21-18-17-16-14(23-19(17)25-20(22)24-18)6-3-7-15(16)26-13-9-8-11-4-1-2-5-12(11)10-13/h1-10H,(H5,21,22,23,24,25) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.60E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Duquesne University
Curated by ChEMBL
| Assay Description
Inhibition of VEGF-induced VEGFR2 phosphorylation in human U251 cells preincubated for 60 mins followed by VEGF induction measured after 10 mins by E... |
Bioorg Med Chem Lett 27: 1602-1607 (2017)
Article DOI: 10.1016/j.bmcl.2017.02.018
BindingDB Entry DOI: 10.7270/Q2P55QR0 |
More data for this
Ligand-Target Pair | |
Epidermal growth factor receptor
(Homo sapiens (Human)) | BDBM50235697

(CHEMBL4088617)Show SMILES Nc1nc(N)c2c(n1)[nH]c1cccc(Sc3cccc4ccccc34)c21 Show InChI InChI=1S/C20H15N5S/c21-18-17-16-13(23-19(17)25-20(22)24-18)8-4-10-15(16)26-14-9-3-6-11-5-1-2-7-12(11)14/h1-10H,(H5,21,22,23,24,25) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 1.67E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Duquesne University
Curated by ChEMBL
| Assay Description
Inhibition of EGF-induced EGFR phosphorylation in human A431 cells preincubated for 60 mins followed by EGF induction measured after 10 mins by ELISA |
Bioorg Med Chem Lett 27: 1602-1607 (2017)
Article DOI: 10.1016/j.bmcl.2017.02.018
BindingDB Entry DOI: 10.7270/Q2P55QR0 |
More data for this
Ligand-Target Pair | |
Vascular endothelial growth factor receptor 2
(Homo sapiens (Human)) | BDBM50235700

(CHEMBL4096329)Show InChI InChI=1S/C17H15N5OS/c1-23-9-5-7-10(8-6-9)24-12-4-2-3-11-13(12)14-15(18)21-17(19)22-16(14)20-11/h2-8H,1H3,(H5,18,19,20,21,22) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 1.82E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Duquesne University
Curated by ChEMBL
| Assay Description
Inhibition of VEGF-induced VEGFR2 phosphorylation in human U251 cells preincubated for 60 mins followed by VEGF induction measured after 10 mins by E... |
Bioorg Med Chem Lett 27: 1602-1607 (2017)
Article DOI: 10.1016/j.bmcl.2017.02.018
BindingDB Entry DOI: 10.7270/Q2P55QR0 |
More data for this
Ligand-Target Pair | |
Vascular endothelial growth factor receptor 2
(Homo sapiens (Human)) | BDBM50235697

(CHEMBL4088617)Show SMILES Nc1nc(N)c2c(n1)[nH]c1cccc(Sc3cccc4ccccc34)c21 Show InChI InChI=1S/C20H15N5S/c21-18-17-16-13(23-19(17)25-20(22)24-18)8-4-10-15(16)26-14-9-3-6-11-5-1-2-7-12(11)14/h1-10H,(H5,21,22,23,24,25) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 1.93E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Duquesne University
Curated by ChEMBL
| Assay Description
Inhibition of VEGF-induced VEGFR2 phosphorylation in human U251 cells preincubated for 60 mins followed by VEGF induction measured after 10 mins by E... |
Bioorg Med Chem Lett 27: 1602-1607 (2017)
Article DOI: 10.1016/j.bmcl.2017.02.018
BindingDB Entry DOI: 10.7270/Q2P55QR0 |
More data for this
Ligand-Target Pair | |
Platelet-derived growth factor receptor beta
(Homo sapiens (Human)) | BDBM50235698

(CHEMBL4069070)Show SMILES COc1ccc(OC)c(Sc2cccc3[nH]c4nc(N)nc(N)c4c23)c1 Show InChI InChI=1S/C18H17N5O2S/c1-24-9-6-7-11(25-2)13(8-9)26-12-5-3-4-10-14(12)15-16(19)22-18(20)23-17(15)21-10/h3-8H,1-2H3,(H5,19,20,21,22,23) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | >2.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Duquesne University
Curated by ChEMBL
| Assay Description
Ability to compete for binding to Class II MHC using ELISA assay. |
Bioorg Med Chem Lett 27: 1602-1607 (2017)
Article DOI: 10.1016/j.bmcl.2017.02.018
BindingDB Entry DOI: 10.7270/Q2P55QR0 |
More data for this
Ligand-Target Pair | |
Platelet-derived growth factor receptor beta
(Homo sapiens (Human)) | BDBM50235699

(CHEMBL4079829)Show SMILES Nc1nc(N)c2c(n1)[nH]c1cccc(Sc3ccc(Cl)cc3)c21 Show InChI InChI=1S/C16H12ClN5S/c17-8-4-6-9(7-5-8)23-11-3-1-2-10-12(11)13-14(18)21-16(19)22-15(13)20-10/h1-7H,(H5,18,19,20,21,22) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | >2.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Duquesne University
Curated by ChEMBL
| Assay Description
Compound was evaluated for its inhibitory activity against Mevalonate 5-pyrophosphate decarboxylase |
Bioorg Med Chem Lett 27: 1602-1607 (2017)
Article DOI: 10.1016/j.bmcl.2017.02.018
BindingDB Entry DOI: 10.7270/Q2P55QR0 |
More data for this
Ligand-Target Pair | |
Vascular endothelial growth factor receptor 2
(Homo sapiens (Human)) | BDBM50235699

(CHEMBL4079829)Show SMILES Nc1nc(N)c2c(n1)[nH]c1cccc(Sc3ccc(Cl)cc3)c21 Show InChI InChI=1S/C16H12ClN5S/c17-8-4-6-9(7-5-8)23-11-3-1-2-10-12(11)13-14(18)21-16(19)22-15(13)20-10/h1-7H,(H5,18,19,20,21,22) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | >5.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Duquesne University
Curated by ChEMBL
| Assay Description
Inhibition of VEGF-induced VEGFR2 phosphorylation in human U251 cells preincubated for 60 mins followed by VEGF induction measured after 10 mins by E... |
Bioorg Med Chem Lett 27: 1602-1607 (2017)
Article DOI: 10.1016/j.bmcl.2017.02.018
BindingDB Entry DOI: 10.7270/Q2P55QR0 |
More data for this
Ligand-Target Pair | |
Dihydrofolate reductase
(Homo sapiens (Human)) | BDBM18069

(5-[(3,4,5-trimethoxyphenyl)methyl]pyrimidine-2,4-d...)Show InChI InChI=1S/C14H18N4O3/c1-19-10-5-8(6-11(20-2)12(10)21-3)4-9-7-17-14(16)18-13(9)15/h5-7H,4H2,1-3H3,(H4,15,16,17,18) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| DrugBank
PDB
Article
PubMed
| n/a | n/a | 6.80E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Duquesne University
Curated by ChEMBL
| Assay Description
Inhibition of human DHFR using dihydrofolate as substrate by spectrophotometric method |
Bioorg Med Chem Lett 27: 1602-1607 (2017)
Article DOI: 10.1016/j.bmcl.2017.02.018
BindingDB Entry DOI: 10.7270/Q2P55QR0 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data