 Found 10 hits Enz. Inhib. hit(s) with all data for entry = 50049253
Found 10 hits Enz. Inhib. hit(s) with all data for entry = 50049253 Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Serine/threonine-protein kinase mTOR
(Homo sapiens (Human)) | BDBM50343759
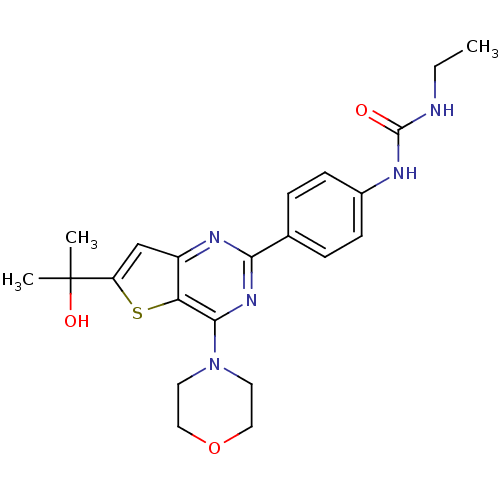
(1-ethyl-3-(4-(6-(2-hydroxypropan-2-yl)-4-morpholin...)Show SMILES CCNC(=O)Nc1ccc(cc1)-c1nc(N2CCOCC2)c2sc(cc2n1)C(C)(C)O Show InChI InChI=1S/C22H27N5O3S/c1-4-23-21(28)24-15-7-5-14(6-8-15)19-25-16-13-17(22(2,3)29)31-18(16)20(26-19)27-9-11-30-12-10-27/h5-8,13,29H,4,9-12H2,1-3H3,(H2,23,24,28) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Peking University
Curated by ChEMBL
| Assay Description
Inhibition of human N-terminal FLAG-tagged mTOR (1362-end residues) in presence of [gamma33P]ATP after 40 mins |
Eur J Med Chem 129: 135-150 (2017)
Article DOI: 10.1016/j.ejmech.2017.02.015
BindingDB Entry DOI: 10.7270/Q2QN692K |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase mTOR
(Homo sapiens (Human)) | BDBM50348452
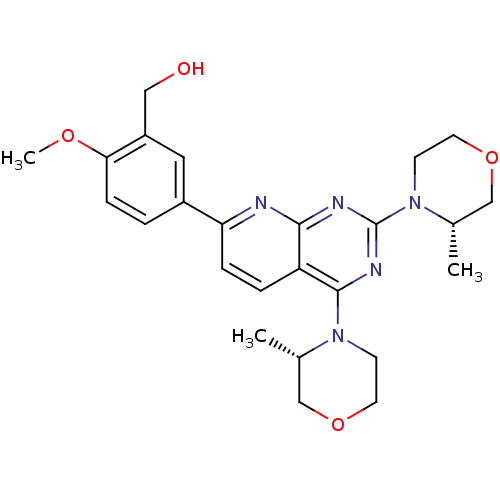
(AZD-8055 | CHEMBL1801204 | US9102670, 1a)Show SMILES COc1ccc(cc1CO)-c1ccc2c(nc(nc2n1)N1CCOC[C@@H]1C)N1CCOC[C@@H]1C |r| Show InChI InChI=1S/C25H31N5O4/c1-16-14-33-10-8-29(16)24-20-5-6-21(18-4-7-22(32-3)19(12-18)13-31)26-23(20)27-25(28-24)30-9-11-34-15-17(30)2/h4-7,12,16-17,31H,8-11,13-15H2,1-3H3/t16-,17-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
Peking University
Curated by ChEMBL
| Assay Description
Inhibition of human N-terminal FLAG-tagged mTOR (1362-end residues) in presence of [gamma33P]ATP after 40 mins |
Eur J Med Chem 129: 135-150 (2017)
Article DOI: 10.1016/j.ejmech.2017.02.015
BindingDB Entry DOI: 10.7270/Q2QN692K |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase mTOR
(Homo sapiens (Human)) | BDBM35615
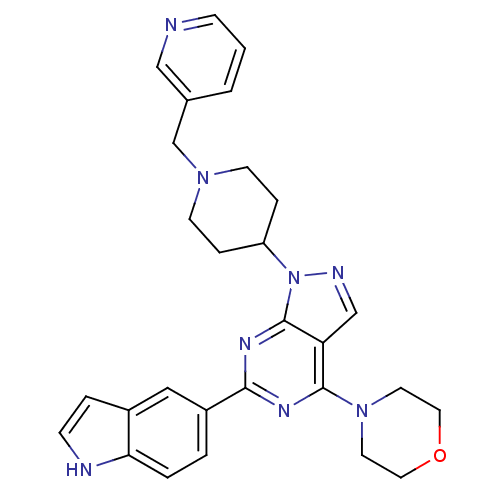
(pyrazolo pyrimidine, 5u)Show SMILES C(N1CCC(CC1)n1ncc2c(nc(nc12)-c1ccc2[nH]ccc2c1)N1CCOCC1)c1cccnc1 Show InChI InChI=1S/C28H30N8O/c1-2-20(17-29-8-1)19-34-10-6-23(7-11-34)36-28-24(18-31-36)27(35-12-14-37-15-13-35)32-26(33-28)22-3-4-25-21(16-22)5-9-30-25/h1-5,8-9,16-18,23,30H,6-7,10-15,19H2 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 9 | n/a | n/a | n/a | n/a | n/a | n/a |
Peking University
Curated by ChEMBL
| Assay Description
Inhibition of mTOR (unknown origin) |
Eur J Med Chem 129: 135-150 (2017)
Article DOI: 10.1016/j.ejmech.2017.02.015
BindingDB Entry DOI: 10.7270/Q2QN692K |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase mTOR
(Homo sapiens (Human)) | BDBM25045

(3-(4-morpholin-4-ylpyrido[2,3]furo[2,4-b]pyrimidin...)Show InChI InChI=1S/C19H16N4O3/c24-13-4-1-3-12(11-13)17-21-15-14-5-2-6-20-19(14)26-16(15)18(22-17)23-7-9-25-10-8-23/h1-6,11,24H,7-10H2 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
Article
PubMed
| n/a | n/a | 31 | n/a | n/a | n/a | n/a | n/a | n/a |
Peking University
Curated by ChEMBL
| Assay Description
In vitro agonist potency was evaluated in HEK293 cells transfected with human melanocortin receptor (hMC1R) |
Eur J Med Chem 129: 135-150 (2017)
Article DOI: 10.1016/j.ejmech.2017.02.015
BindingDB Entry DOI: 10.7270/Q2QN692K |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Phosphatidylinositol 3-kinase regulatory subunit alpha/4,5-bisphosphate 3-kinase catalytic subunit alpha isoform
(Homo sapiens (Human)) | BDBM25045

(3-(4-morpholin-4-ylpyrido[2,3]furo[2,4-b]pyrimidin...)Show InChI InChI=1S/C19H16N4O3/c24-13-4-1-3-12(11-13)17-21-15-14-5-2-6-20-19(14)26-16(15)18(22-17)23-7-9-25-10-8-23/h1-6,11,24H,7-10H2 | PDB
UniProtKB/SwissProt
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
Article
PubMed
| n/a | n/a | 40 | n/a | n/a | n/a | n/a | n/a | n/a |
Peking University
Curated by ChEMBL
| Assay Description
In vitro agonist potency was evaluated in HEK293 cells transfected with human melanocortin receptor (hMC1R) |
Eur J Med Chem 129: 135-150 (2017)
Article DOI: 10.1016/j.ejmech.2017.02.015
BindingDB Entry DOI: 10.7270/Q2QN692K |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Serine/threonine-protein kinase mTOR
(Homo sapiens (Human)) | BDBM50237534
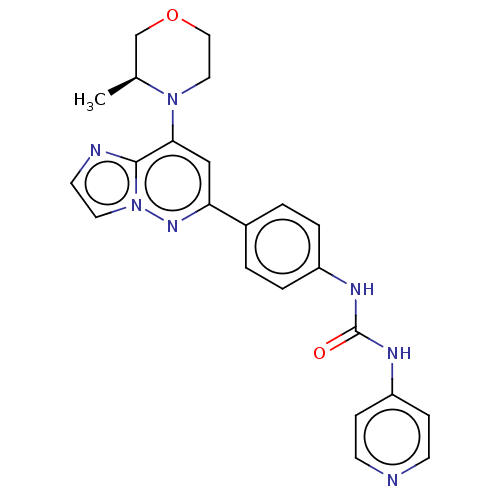
(CHEMBL4091820)Show SMILES C[C@H]1COCCN1c1cc(nn2ccnc12)-c1ccc(NC(=O)Nc2ccncc2)cc1 |r| Show InChI InChI=1S/C23H23N7O2/c1-16-15-32-13-12-29(16)21-14-20(28-30-11-10-25-22(21)30)17-2-4-18(5-3-17)26-23(31)27-19-6-8-24-9-7-19/h2-11,14,16H,12-13,15H2,1H3,(H2,24,26,27,31)/t16-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 62 | n/a | n/a | n/a | n/a | n/a | n/a |
Peking University
Curated by ChEMBL
| Assay Description
Inhibition of human N-terminal FLAG-tagged mTOR (1362-end residues) in presence of [gamma33P]ATP after 40 mins |
Eur J Med Chem 129: 135-150 (2017)
Article DOI: 10.1016/j.ejmech.2017.02.015
BindingDB Entry DOI: 10.7270/Q2QN692K |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase mTOR
(Homo sapiens (Human)) | BDBM50237533
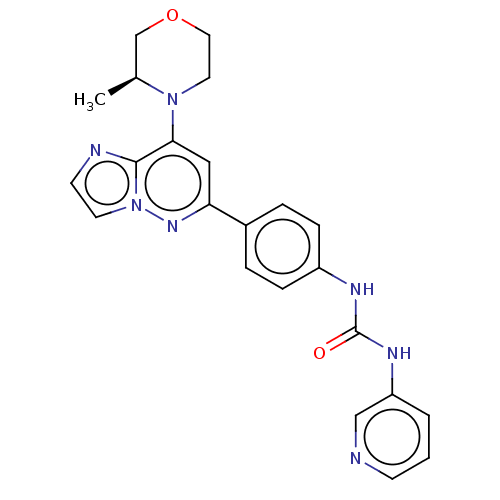
(CHEMBL4062346)Show SMILES C[C@H]1COCCN1c1cc(nn2ccnc12)-c1ccc(NC(=O)Nc2cccnc2)cc1 |r| Show InChI InChI=1S/C23H23N7O2/c1-16-15-32-12-11-29(16)21-13-20(28-30-10-9-25-22(21)30)17-4-6-18(7-5-17)26-23(31)27-19-3-2-8-24-14-19/h2-10,13-14,16H,11-12,15H2,1H3,(H2,26,27,31)/t16-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 67 | n/a | n/a | n/a | n/a | n/a | n/a |
Peking University
Curated by ChEMBL
| Assay Description
Inhibition of human N-terminal FLAG-tagged mTOR (1362-end residues) in presence of [gamma33P]ATP after 40 mins |
Eur J Med Chem 129: 135-150 (2017)
Article DOI: 10.1016/j.ejmech.2017.02.015
BindingDB Entry DOI: 10.7270/Q2QN692K |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 3-kinase regulatory subunit alpha/4,5-bisphosphate 3-kinase catalytic subunit alpha isoform
(Homo sapiens (Human)) | BDBM50237534

(CHEMBL4091820)Show SMILES C[C@H]1COCCN1c1cc(nn2ccnc12)-c1ccc(NC(=O)Nc2ccncc2)cc1 |r| Show InChI InChI=1S/C23H23N7O2/c1-16-15-32-13-12-29(16)21-14-20(28-30-11-10-25-22(21)30)17-2-4-18(5-3-17)26-23(31)27-19-6-8-24-9-7-19/h2-11,14,16H,12-13,15H2,1H3,(H2,24,26,27,31)/t16-/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 3.11E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Peking University
Curated by ChEMBL
| Assay Description
Inhibition of human PI3K p110alpha/p85alpha using phosphatidylinositol 4,5-bisphosphate as substrate after 30 mins by HTRF assay |
Eur J Med Chem 129: 135-150 (2017)
Article DOI: 10.1016/j.ejmech.2017.02.015
BindingDB Entry DOI: 10.7270/Q2QN692K |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 3-kinase regulatory subunit alpha/4,5-bisphosphate 3-kinase catalytic subunit alpha isoform
(Homo sapiens (Human)) | BDBM50237533

(CHEMBL4062346)Show SMILES C[C@H]1COCCN1c1cc(nn2ccnc12)-c1ccc(NC(=O)Nc2cccnc2)cc1 |r| Show InChI InChI=1S/C23H23N7O2/c1-16-15-32-12-11-29(16)21-13-20(28-30-10-9-25-22(21)30)17-4-6-18(7-5-17)26-23(31)27-19-3-2-8-24-14-19/h2-10,13-14,16H,11-12,15H2,1H3,(H2,26,27,31)/t16-/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 4.42E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Peking University
Curated by ChEMBL
| Assay Description
In vitro agonist potency was evaluated in HEK293 cells transfected with human melanocortin receptor (hMC4R) |
Eur J Med Chem 129: 135-150 (2017)
Article DOI: 10.1016/j.ejmech.2017.02.015
BindingDB Entry DOI: 10.7270/Q2QN692K |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 3-kinase regulatory subunit alpha/4,5-bisphosphate 3-kinase catalytic subunit alpha isoform
(Homo sapiens (Human)) | BDBM50348452

(AZD-8055 | CHEMBL1801204 | US9102670, 1a)Show SMILES COc1ccc(cc1CO)-c1ccc2c(nc(nc2n1)N1CCOC[C@@H]1C)N1CCOC[C@@H]1C |r| Show InChI InChI=1S/C25H31N5O4/c1-16-14-33-10-8-29(16)24-20-5-6-21(18-4-7-22(32-3)19(12-18)13-31)26-23(20)27-25(28-24)30-9-11-34-15-17(30)2/h4-7,12,16-17,31H,8-11,13-15H2,1-3H3/t16-,17-/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 5.58E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Peking University
Curated by ChEMBL
| Assay Description
Inhibition of human PI3K p110alpha/p85alpha using phosphatidylinositol 4,5-bisphosphate as substrate after 30 mins by HTRF assay |
Eur J Med Chem 129: 135-150 (2017)
Article DOI: 10.1016/j.ejmech.2017.02.015
BindingDB Entry DOI: 10.7270/Q2QN692K |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data