

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Delta-type opioid receptor (Rattus norvegicus (rat)) | BDBM50001683 (13-[2-Amino-3-(4-hydroxy-phenyl)-propionylamino]-7...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Arizona Curated by ChEMBL | Assay Description Binding potency of compound in competition with [3H]DPDPE for Opioid receptor delta 1 tested in rat brain membrane | J Med Chem 32: 638-43 (1989) BindingDB Entry DOI: 10.7270/Q2DN45N0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Rattus norvegicus (rat)) | BDBM50001683 (13-[2-Amino-3-(4-hydroxy-phenyl)-propionylamino]-7...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 385 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Arizona Curated by ChEMBL | Assay Description The ability to displace [3H]naloxone from the Opioid receptor mu 1 isolated from rat brain membrane. | J Med Chem 32: 638-43 (1989) BindingDB Entry DOI: 10.7270/Q2DN45N0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Rattus norvegicus (rat)) | BDBM50452603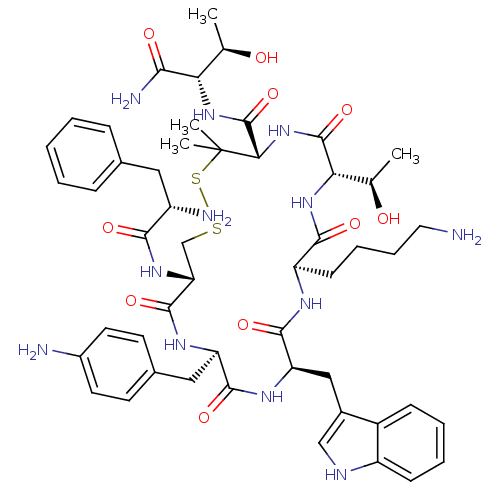 (CHEMBL2371213) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 500 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Arizona Curated by ChEMBL | Assay Description Binding potency of compound in competition with [3H]CTOP for Opioid receptor mu 1 tested in rat brain membrane | J Med Chem 32: 638-43 (1989) BindingDB Entry DOI: 10.7270/Q2DN45N0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Rattus norvegicus (rat)) | BDBM50016857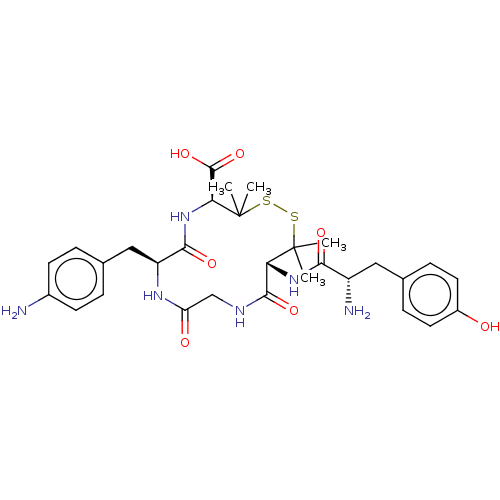 (13-[2-Amino-3-(4-hydroxy-phenyl)-propionylamino]-7...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 1.05E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Arizona Curated by ChEMBL | Assay Description Binding potency of compound in competition with [3H]CTOP for Opioid receptor mu 1 tested in rat brain membrane | J Med Chem 32: 638-43 (1989) BindingDB Entry DOI: 10.7270/Q2DN45N0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Delta-type opioid receptor (Rattus norvegicus (rat)) | BDBM50016857 (13-[2-Amino-3-(4-hydroxy-phenyl)-propionylamino]-7...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 1.05E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Arizona Curated by ChEMBL | Assay Description Binding potency of compound in competition with [3H]DPDPE for Opioid receptor delta 1 tested in rat brain membrane | J Med Chem 32: 638-43 (1989) BindingDB Entry DOI: 10.7270/Q2DN45N0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Delta-type opioid receptor (Rattus norvegicus (rat)) | BDBM50016853 (2-{[10-(4-Amino-butyl)-19-(2-amino-3-phenyl-propio...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 3.61E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Arizona Curated by ChEMBL | Assay Description Binding potency of compound in competition with [3H]DPDPE for Opioid receptor delta 1 tested in rat brain membrane | J Med Chem 32: 638-43 (1989) BindingDB Entry DOI: 10.7270/Q2DN45N0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Rattus norvegicus (rat)) | BDBM50016853 (2-{[10-(4-Amino-butyl)-19-(2-amino-3-phenyl-propio...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 3.62E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Arizona Curated by ChEMBL | Assay Description Binding potency of compound in competition with [3H]CTOP for Opioid receptor mu 1 tested in rat brain membrane | J Med Chem 32: 638-43 (1989) BindingDB Entry DOI: 10.7270/Q2DN45N0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Delta-type opioid receptor (Rattus norvegicus (rat)) | BDBM50016854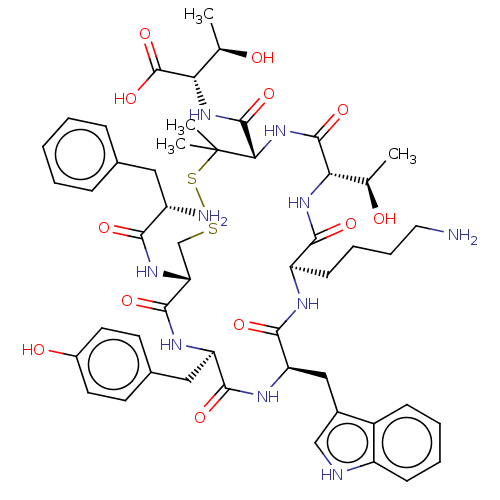 (2-{[10-(4-Amino-butyl)-19-(2-amino-3-phenyl-propio...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 8.40E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Arizona Curated by ChEMBL | Assay Description Binding potency of compound in competition with [3H]DPDPE for Opioid receptor delta 1 tested in rat brain membrane | J Med Chem 32: 638-43 (1989) BindingDB Entry DOI: 10.7270/Q2DN45N0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Rattus norvegicus (rat)) | BDBM50016854 (2-{[10-(4-Amino-butyl)-19-(2-amino-3-phenyl-propio...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 8.40E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Arizona Curated by ChEMBL | Assay Description Binding potency of compound in competition with [3H]CTOP for Opioid receptor mu 1 tested in rat brain membrane | J Med Chem 32: 638-43 (1989) BindingDB Entry DOI: 10.7270/Q2DN45N0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Delta-type opioid receptor (Rattus norvegicus (rat)) | BDBM50452603 (CHEMBL2371213) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Arizona Curated by ChEMBL | Assay Description Binding potency of compound in competition with [3H]DPDPE for Opioid receptor delta 1 tested in rat brain membrane | J Med Chem 32: 638-43 (1989) BindingDB Entry DOI: 10.7270/Q2DN45N0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Delta-type opioid receptor (Rattus norvegicus (rat)) | BDBM50016858 (2-{[16-(4-Amino-benzyl)-10-(4-amino-butyl)-19-(2-a...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 2.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Arizona Curated by ChEMBL | Assay Description Binding potency of compound in competition with [3H]DPDPE for Opioid receptor delta 1 tested in rat brain membrane | J Med Chem 32: 638-43 (1989) BindingDB Entry DOI: 10.7270/Q2DN45N0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Rattus norvegicus (rat)) | BDBM50016858 (2-{[16-(4-Amino-benzyl)-10-(4-amino-butyl)-19-(2-a...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 2.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Arizona Curated by ChEMBL | Assay Description Binding potency of compound in competition with [3H]CTOP for Opioid receptor mu 1 tested in rat brain membrane | J Med Chem 32: 638-43 (1989) BindingDB Entry DOI: 10.7270/Q2DN45N0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||