

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Aromatase (Homo sapiens (Human)) | BDBM50014307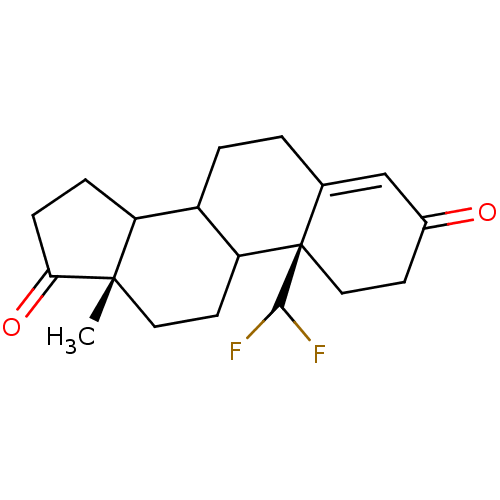 (10-Difluoromethyl-13-methyl-1,6,7,8,9,10,11,12,13,...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johns Hopkins University School of Medicine Curated by ChEMBL | Assay Description Binding affinity was measured on Cytochrome P450 19A1 | J Med Chem 33: 2933-42 (1990) BindingDB Entry DOI: 10.7270/Q2VM4CW9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aromatase (Homo sapiens (Human)) | BDBM50011771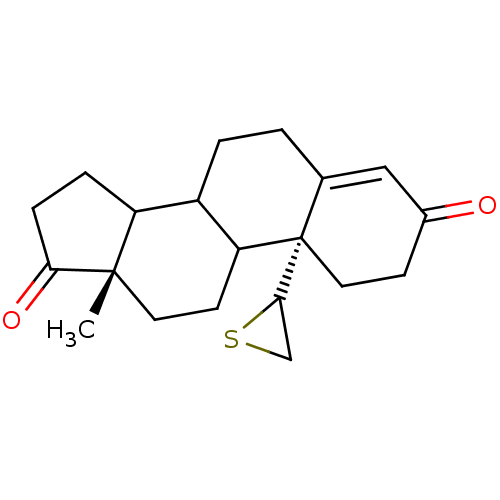 (13-Methyl-10-thiiranyl-1,6,7,8,9,10,11,12,13,14,15...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johns Hopkins University School of Medicine Curated by ChEMBL | Assay Description Binding affinity was measured on Cytochrome P450 19A1 | J Med Chem 33: 2933-42 (1990) BindingDB Entry DOI: 10.7270/Q2VM4CW9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aromatase (Homo sapiens (Human)) | BDBM10044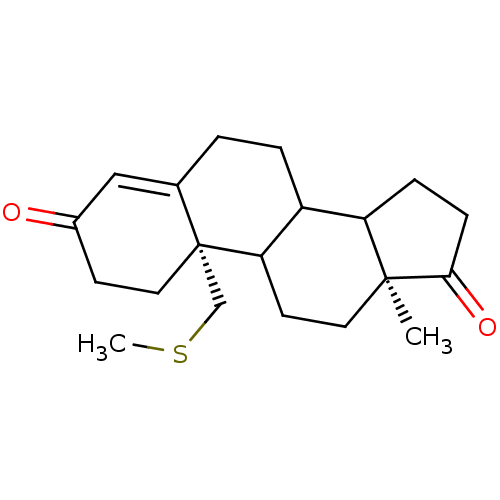 ((2S,15S)-15-methyl-2-[(methylsulfanyl)methyl]tetra...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johns Hopkins University School of Medicine Curated by ChEMBL | Assay Description Binding affinity was measured on Cytochrome P450 19A1 | J Med Chem 33: 2933-42 (1990) BindingDB Entry DOI: 10.7270/Q2VM4CW9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aromatase (Homo sapiens (Human)) | BDBM50014316 (7-(4-Azido-phenylsulfanyl)-10,13-dimethyl-1,6,7,8,...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 1.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johns Hopkins University School of Medicine Curated by ChEMBL | Assay Description Binding affinity was measured on Cytochrome P450 19A1 | J Med Chem 33: 2933-42 (1990) BindingDB Entry DOI: 10.7270/Q2VM4CW9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aromatase (Homo sapiens (Human)) | BDBM50014316 (7-(4-Azido-phenylsulfanyl)-10,13-dimethyl-1,6,7,8,...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 1.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johns Hopkins University School of Medicine Curated by ChEMBL | Assay Description Binding affinity was measured on Cytochrome P450 19A1 | J Med Chem 33: 2933-42 (1990) BindingDB Entry DOI: 10.7270/Q2VM4CW9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aromatase (Homo sapiens (Human)) | BDBM8611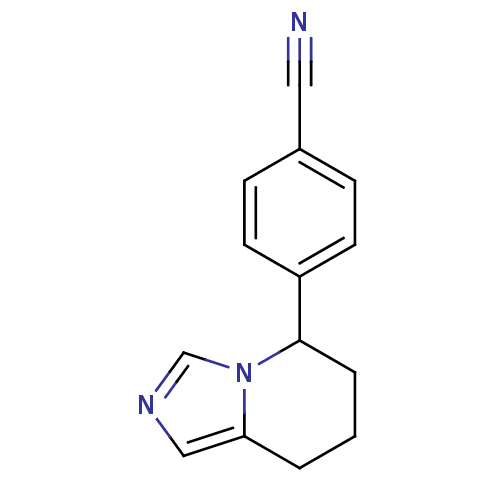 (4-{5H,6H,7H,8H-imidazo[1,5-a]pyridin-5-yl}benzonit...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid PDB UniChem Patents Similars | PubMed | 1.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johns Hopkins University School of Medicine Curated by ChEMBL | Assay Description Binding affinity was measured on Cytochrome P450 19A1 | J Med Chem 33: 2933-42 (1990) BindingDB Entry DOI: 10.7270/Q2VM4CW9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aromatase (Homo sapiens (Human)) | BDBM50014299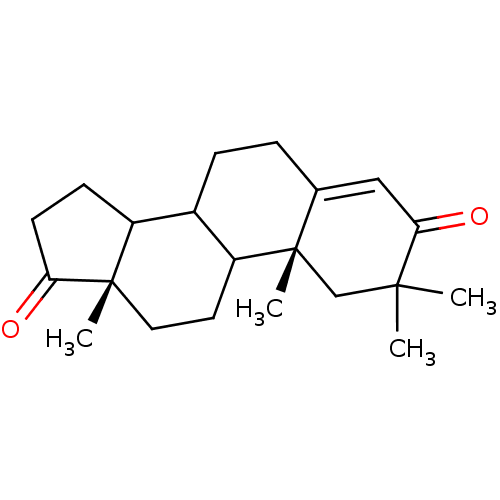 (2,2,10,13-Tetramethyl-1,6,7,8,9,10,11,12,13,14,15,...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 2.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johns Hopkins University School of Medicine Curated by ChEMBL | Assay Description Binding affinity was measured on Cytochrome P450 19A1 | J Med Chem 33: 2933-42 (1990) BindingDB Entry DOI: 10.7270/Q2VM4CW9 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (docked) | ||||||||||||
| Aromatase (Homo sapiens (Human)) | BDBM50014310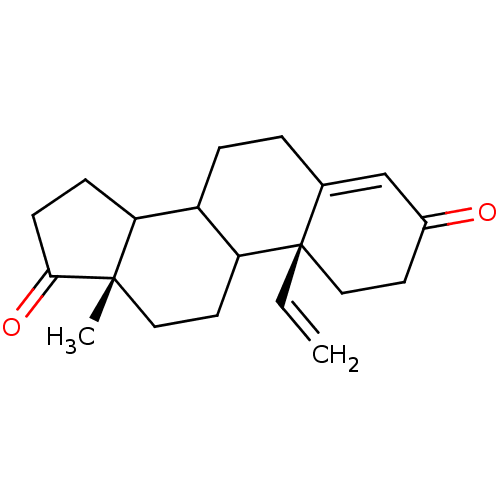 (13-Methyl-10-vinyl-1,6,7,8,9,10,11,12,13,14,15,16-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johns Hopkins University School of Medicine Curated by ChEMBL | Assay Description Binding affinity was measured on Cytochrome P450 19A1 | J Med Chem 33: 2933-42 (1990) BindingDB Entry DOI: 10.7270/Q2VM4CW9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aromatase (Homo sapiens (Human)) | BDBM50014302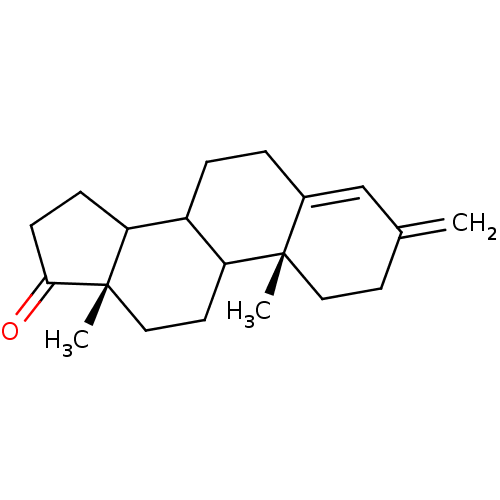 (10,13-Dimethyl-3-methylene-1,2,3,6,7,8,9,10,11,12,...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 4.70 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johns Hopkins University School of Medicine Curated by ChEMBL | Assay Description Binding affinity was measured on Cytochrome P450 19A1 | J Med Chem 33: 2933-42 (1990) BindingDB Entry DOI: 10.7270/Q2VM4CW9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aromatase (Homo sapiens (Human)) | BDBM50014319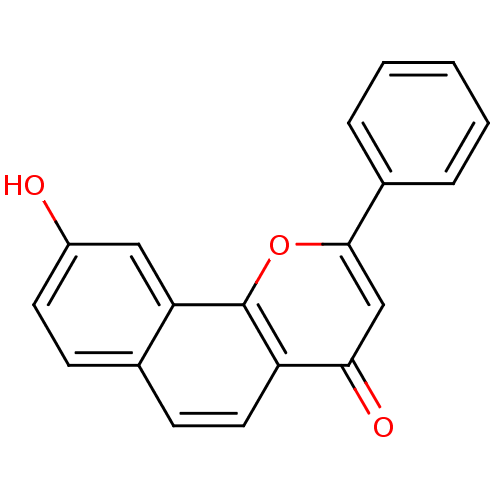 (9-Hydroxy-2-phenyl-benzo[h]chromen-4-one | CHEMBL3...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johns Hopkins University School of Medicine Curated by ChEMBL | Assay Description Binding affinity was measured on Cytochrome P450 19A1 | J Med Chem 33: 2933-42 (1990) BindingDB Entry DOI: 10.7270/Q2VM4CW9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aromatase (Homo sapiens (Human)) | BDBM50011774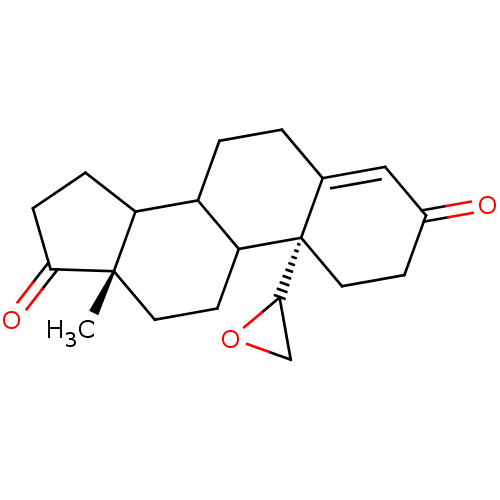 (13-Methyl-10-oxiranyl-1,6,7,8,9,10,11,12,13,14,15,...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 7 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johns Hopkins University School of Medicine Curated by ChEMBL | Assay Description Binding affinity was measured on Cytochrome P450 19A1 | J Med Chem 33: 2933-42 (1990) BindingDB Entry DOI: 10.7270/Q2VM4CW9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aromatase (Homo sapiens (Human)) | BDBM50014311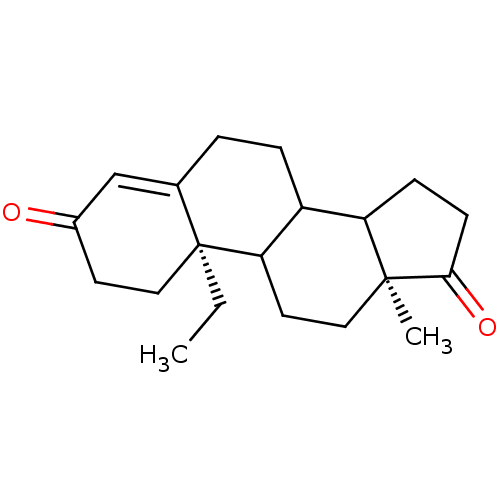 (10-Ethyl-13-methyl-1,6,7,8,9,10,11,12,13,14,15,16-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 9 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johns Hopkins University School of Medicine Curated by ChEMBL | Assay Description Binding affinity was measured on Cytochrome P450 19A1 | J Med Chem 33: 2933-42 (1990) BindingDB Entry DOI: 10.7270/Q2VM4CW9 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (docked) | ||||||||||||
| Aromatase (Homo sapiens (Human)) | BDBM50014309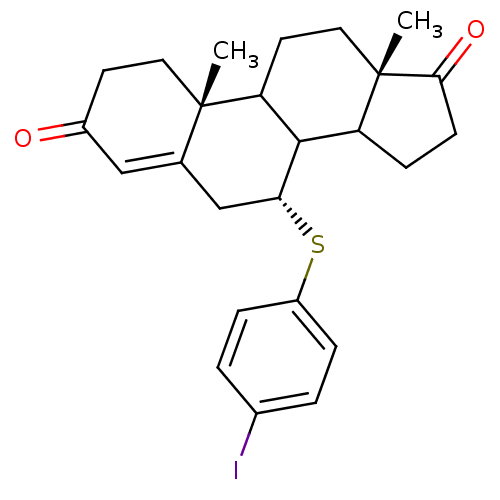 (7-(4-Iodo-phenylsulfanyl)-10,13-dimethyl-1,6,7,8,9...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 12 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johns Hopkins University School of Medicine Curated by ChEMBL | Assay Description Binding affinity was measured on Cytochrome P450 19A1 | J Med Chem 33: 2933-42 (1990) BindingDB Entry DOI: 10.7270/Q2VM4CW9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aromatase (Homo sapiens (Human)) | BDBM50014323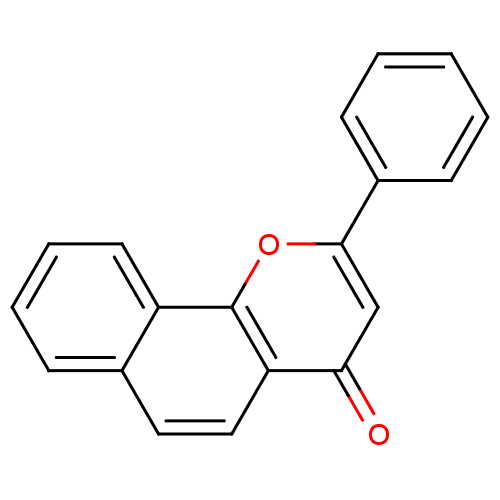 (2-PHENYL-4H-BENZO[H]CHROMEN-4-ONE | 2-Phenyl-benzo...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE MMDB PC cid PC sid PDB UniChem Patents Similars | PubMed | 20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johns Hopkins University School of Medicine Curated by ChEMBL | Assay Description Binding affinity was measured on Cytochrome P450 19A1 | J Med Chem 33: 2933-42 (1990) BindingDB Entry DOI: 10.7270/Q2VM4CW9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aromatase (Rattus norvegicus) | BDBM50014320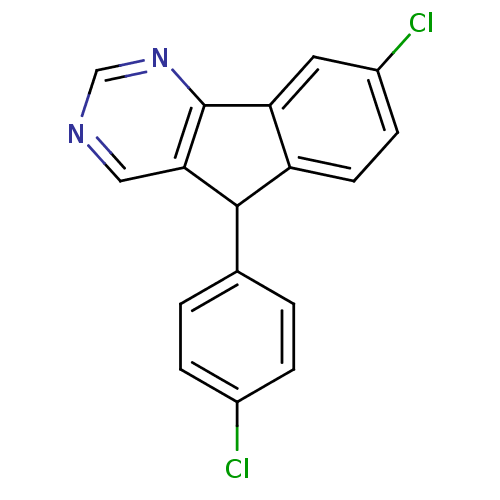 (8-Chloro-5-(4-chloro-phenyl)-5H-indeno[1,2-d]pyrim...) | Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Patents | PubMed | 24 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johns Hopkins University School of Medicine Curated by ChEMBL | Assay Description Binding affinity for Cytochrome P450 19A1 | J Med Chem 33: 2933-42 (1990) BindingDB Entry DOI: 10.7270/Q2VM4CW9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aromatase (Rattus norvegicus) | BDBM50014324 (5-[Bis-(4-chloro-phenyl)-methyl]-pyrimidine | 5-[B...) | Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | PubMed | 29 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johns Hopkins University School of Medicine Curated by ChEMBL | Assay Description Binding affinity for Cytochrome P450 19A1 | J Med Chem 33: 2933-42 (1990) BindingDB Entry DOI: 10.7270/Q2VM4CW9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aromatase (Homo sapiens (Human)) | BDBM50014308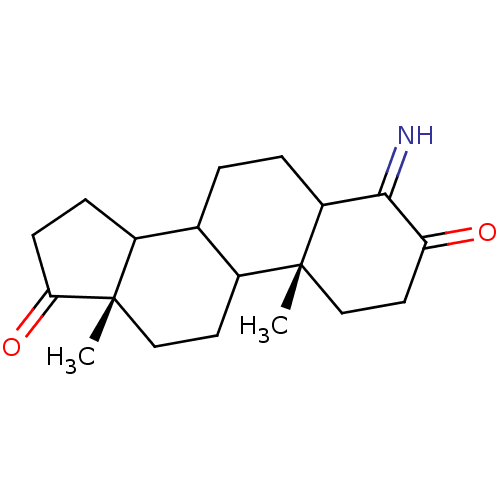 (4-Amino-10,13-dimethyl-1,6,7,8,9,10,11,12,13,14,15...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | PubMed | 37 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johns Hopkins University School of Medicine Curated by ChEMBL | Assay Description Binding affinity was measured on Cytochrome P450 19A1 | J Med Chem 33: 2933-42 (1990) BindingDB Entry DOI: 10.7270/Q2VM4CW9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aromatase (Homo sapiens (Human)) | BDBM50332807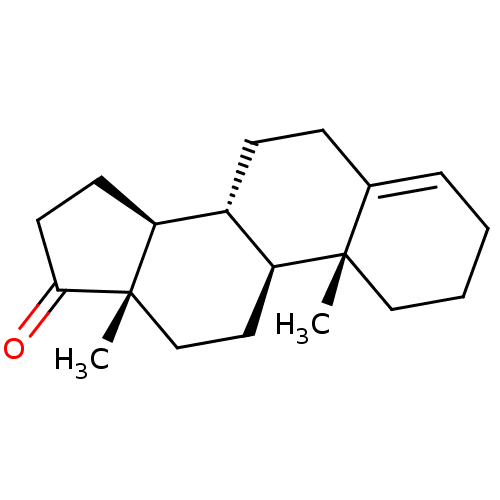 ((8R,9S,10R,13S,14S)-10,13-dimethyl-2,3,7,8,9,10,11...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 37 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johns Hopkins University School of Medicine Curated by ChEMBL | Assay Description Binding affinity was measured on Cytochrome P450 19A1 | J Med Chem 33: 2933-42 (1990) BindingDB Entry DOI: 10.7270/Q2VM4CW9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aromatase (Homo sapiens (Human)) | BDBM50240798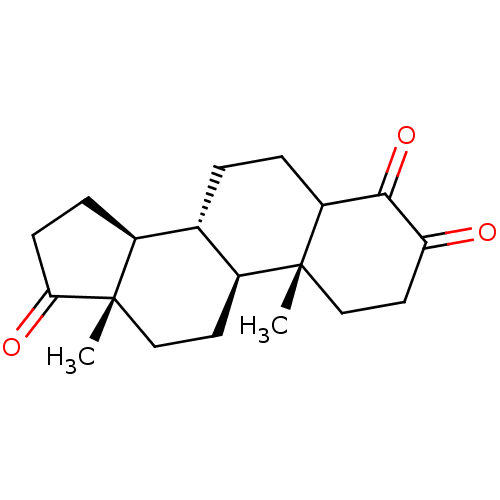 ((8R,9S,10R,13S,14S)-4-Hydroxy-10,13-dimethyl-1,6,7...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 50 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johns Hopkins University School of Medicine Curated by ChEMBL | Assay Description Binding affinity was measured on Cytochrome P450 19A1 | J Med Chem 33: 2933-42 (1990) BindingDB Entry DOI: 10.7270/Q2VM4CW9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aromatase (Homo sapiens (Human)) | BDBM50014312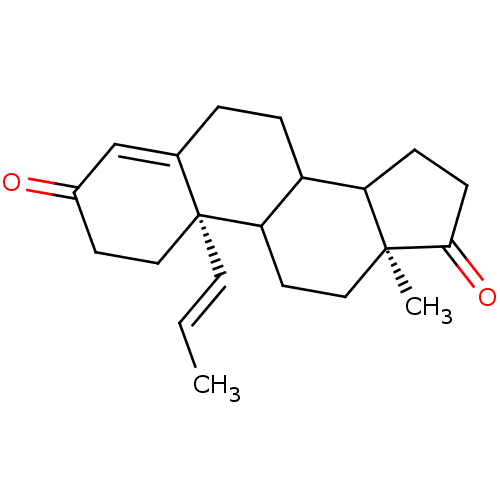 (13-Methyl-10-propenyl-1,6,7,8,9,10,11,12,13,14,15,...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 56 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johns Hopkins University School of Medicine Curated by ChEMBL | Assay Description Binding affinity was measured on Cytochrome P450 19A1 | J Med Chem 33: 2933-42 (1990) BindingDB Entry DOI: 10.7270/Q2VM4CW9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aromatase (Homo sapiens (Human)) | BDBM50011775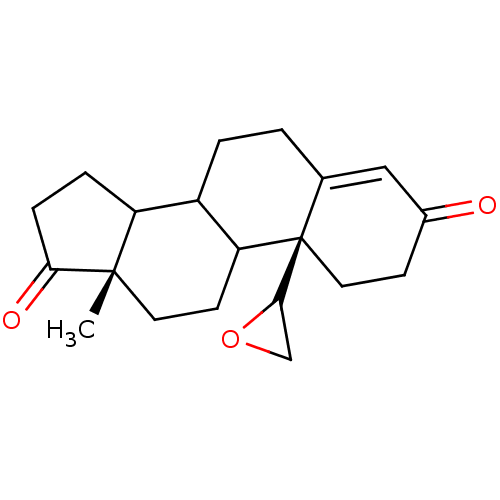 (13-Methyl-10-oxiranyl-1,6,7,8,9,10,11,12,13,14,15,...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 75 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johns Hopkins University School of Medicine Curated by ChEMBL | Assay Description Binding affinity was measured on Cytochrome P450 19A1 | J Med Chem 33: 2933-42 (1990) BindingDB Entry DOI: 10.7270/Q2VM4CW9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aromatase (Homo sapiens (Human)) | BDBM50011770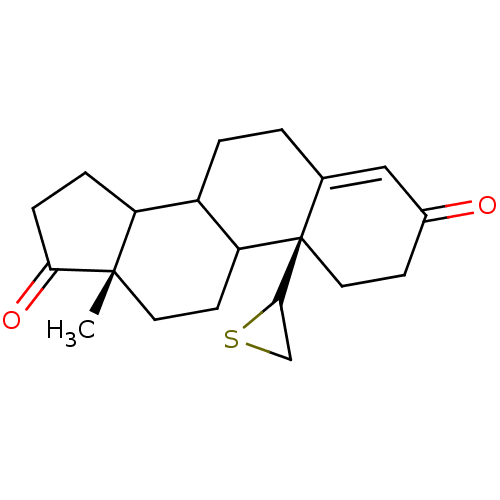 (13-Methyl-10-thiiranyl-1,6,7,8,9,10,11,12,13,14,15...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 75 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johns Hopkins University School of Medicine Curated by ChEMBL | Assay Description Binding affinity was measured on Cytochrome P450 19A1 | J Med Chem 33: 2933-42 (1990) BindingDB Entry DOI: 10.7270/Q2VM4CW9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aromatase (Homo sapiens (Human)) | BDBM50421878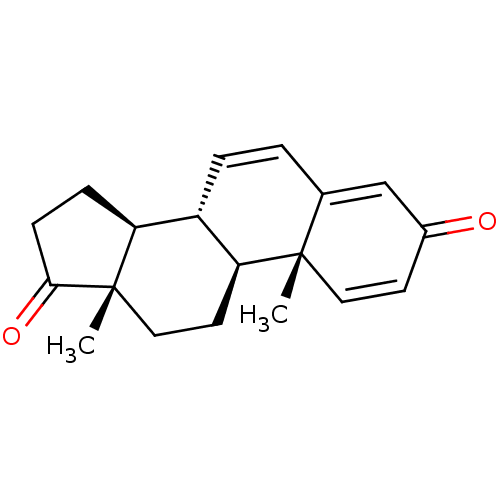 (CHEMBL2311178) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Similars | PubMed | 180 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johns Hopkins University School of Medicine Curated by ChEMBL | Assay Description Binding affinity was measured on Cytochrome P450 19A1 | J Med Chem 33: 2933-42 (1990) BindingDB Entry DOI: 10.7270/Q2VM4CW9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aromatase (Homo sapiens (Human)) | BDBM91718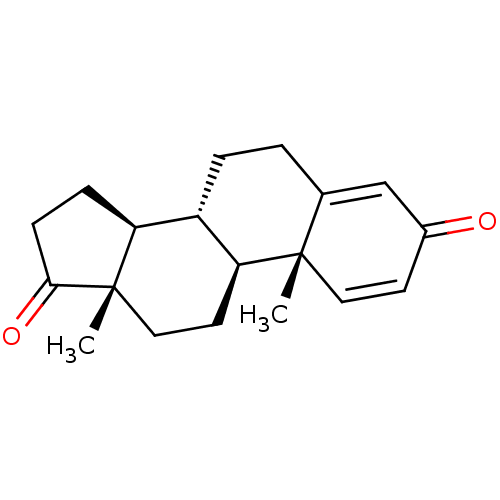 (Androst-1,4-dien-3,17-dione, 7) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase DrugBank MMDB PC cid PC sid PDB UniChem Patents Similars | PubMed | 320 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johns Hopkins University School of Medicine Curated by ChEMBL | Assay Description Binding affinity was measured on Cytochrome P450 19A1 | J Med Chem 33: 2933-42 (1990) BindingDB Entry DOI: 10.7270/Q2VM4CW9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aromatase (Homo sapiens (Human)) | BDBM50014314 (9a,11a-Dimethyl-3a,4,5,9a,9b,10,11,11a-octahydro-3...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 390 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johns Hopkins University School of Medicine Curated by ChEMBL | Assay Description Binding affinity was measured on Cytochrome P450 19A1 | J Med Chem 33: 2933-42 (1990) BindingDB Entry DOI: 10.7270/Q2VM4CW9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aromatase (Homo sapiens (Human)) | BDBM9460 (3-(4-aminophenyl)-3-ethyl-piperidine-2,6-dione | 3...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank KEGG PC cid PC sid PDB UniChem Patents Similars | DrugBank PubMed | 600 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johns Hopkins University School of Medicine Curated by ChEMBL | Assay Description Binding affinity was measured on Cytochrome P450 19A1 | J Med Chem 33: 2933-42 (1990) BindingDB Entry DOI: 10.7270/Q2VM4CW9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aromatase (Homo sapiens (Human)) | BDBM50014301 (10,13-Dimethyl-6,7,8,9,10,11,12,13,14,15,16,17-dod...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 1.10E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johns Hopkins University School of Medicine Curated by ChEMBL | Assay Description Binding affinity was measured on Cytochrome P450 19A1 | J Med Chem 33: 2933-42 (1990) BindingDB Entry DOI: 10.7270/Q2VM4CW9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aromatase (Homo sapiens (Human)) | BDBM50014322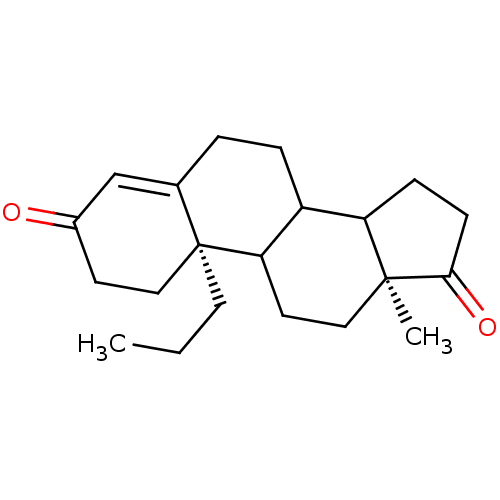 (13-Methyl-10-propyl-1,6,7,8,9,10,11,12,13,14,15,16...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 3.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johns Hopkins University School of Medicine Curated by ChEMBL | Assay Description Binding affinity was measured on Cytochrome P450 19A1 | J Med Chem 33: 2933-42 (1990) BindingDB Entry DOI: 10.7270/Q2VM4CW9 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (docked) | ||||||||||||
| Aromatase (Homo sapiens (Human)) | BDBM50014318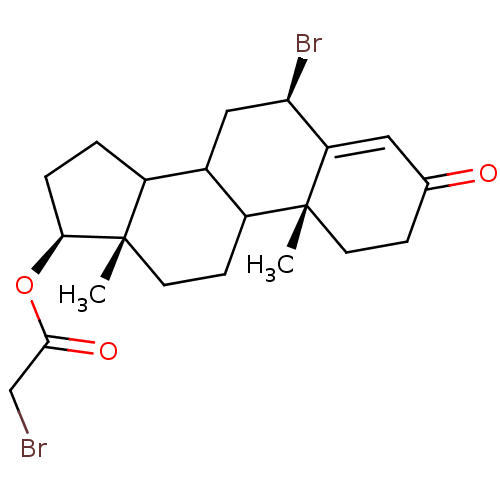 (Bromo-acetic acid 6-bromo-10,13-dimethyl-3-oxo-2,3...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | PubMed | 2.50E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johns Hopkins University School of Medicine Curated by ChEMBL | Assay Description Binding affinity was measured on Cytochrome P450 19A1 | J Med Chem 33: 2933-42 (1990) BindingDB Entry DOI: 10.7270/Q2VM4CW9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aromatase (Homo sapiens (Human)) | BDBM50014304 (2-Bromo-N-[4-(10,13-dimethyl-3,17-dioxo-2,3,6,7,8,...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | PubMed | 3.30E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johns Hopkins University School of Medicine Curated by ChEMBL | Assay Description Binding affinity was measured on Cytochrome P450 19A1 | J Med Chem 33: 2933-42 (1990) BindingDB Entry DOI: 10.7270/Q2VM4CW9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aromatase (Homo sapiens (Human)) | BDBM50014325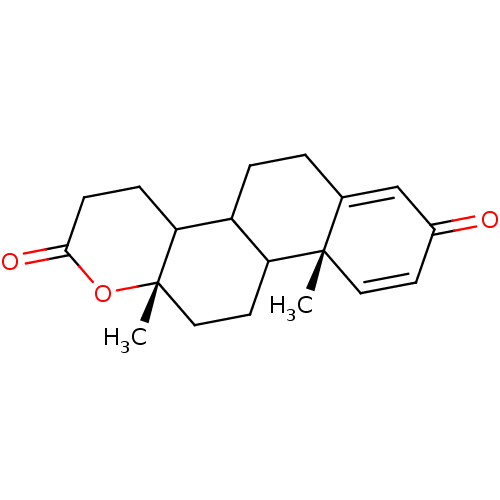 (10a,12a-Dimethyl-3,4,4a,5,6,10a,10b,11,12,12a-deca...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 3.50E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johns Hopkins University School of Medicine Curated by ChEMBL | Assay Description Binding affinity was measured on Cytochrome P450 19A1 | J Med Chem 33: 2933-42 (1990) BindingDB Entry DOI: 10.7270/Q2VM4CW9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aromatase (Homo sapiens (Human)) | BDBM31768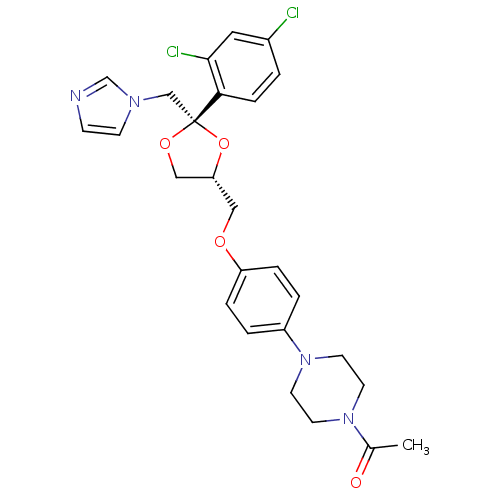 (CHEMBL295698 | Ketoconazole | Nizoral | Panfungol) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE MMDB PC cid PC sid PDB UniChem Patents Similars | PubMed | 6.50E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johns Hopkins University School of Medicine Curated by ChEMBL | Assay Description Binding affinity was measured on Cytochrome P450 19A1 | J Med Chem 33: 2933-42 (1990) BindingDB Entry DOI: 10.7270/Q2VM4CW9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aromatase (Rattus norvegicus) | BDBM50014315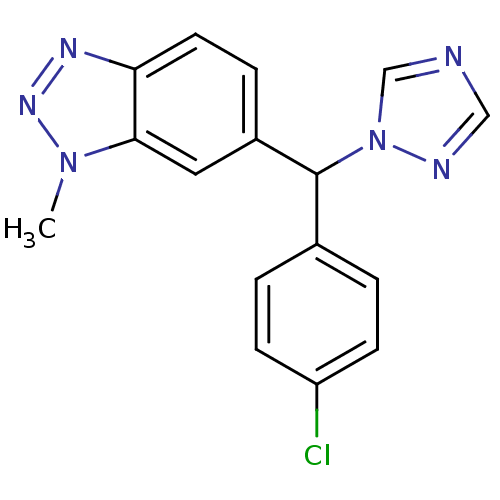 ((rac)-6-((4-chlorophenyl)(1H-1,2,4-triazol-1-yl)me...) | Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
Johns Hopkins University School of Medicine Curated by ChEMBL | Assay Description Binding affinity for Cytochrome P450 19A1 | J Med Chem 33: 2933-42 (1990) BindingDB Entry DOI: 10.7270/Q2VM4CW9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aromatase (Homo sapiens (Human)) | BDBM50014306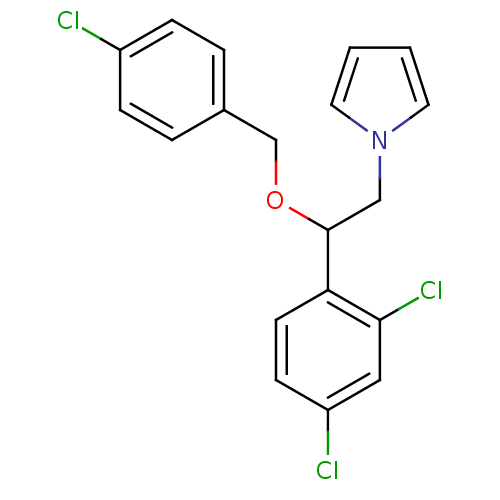 (1-[2-(4-Chloro-benzyloxy)-2-(2,4-dichloro-phenyl)-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents | PubMed | n/a | n/a | 30 | n/a | n/a | n/a | n/a | n/a | n/a |
Johns Hopkins University School of Medicine Curated by ChEMBL | Assay Description Binding affinity for Cytochrome P450 19A1 | J Med Chem 33: 2933-42 (1990) BindingDB Entry DOI: 10.7270/Q2VM4CW9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aromatase (Homo sapiens (Human)) | BDBM50014305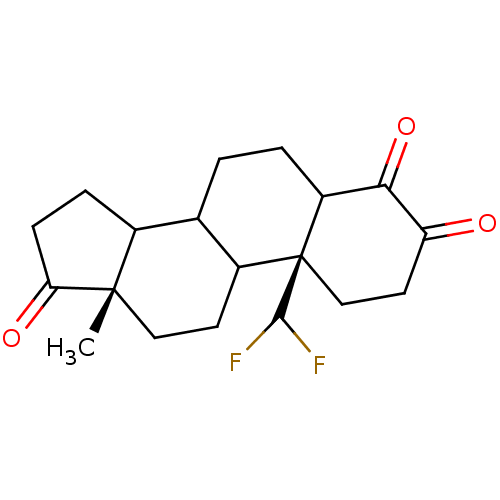 (10-Difluoromethyl-4-hydroxy-13-methyl-1,6,7,8,9,10...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | PubMed | n/a | n/a | 3.30E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Johns Hopkins University School of Medicine Curated by ChEMBL | Assay Description Binding affinity for Cytochrome P450 19A1 | J Med Chem 33: 2933-42 (1990) BindingDB Entry DOI: 10.7270/Q2VM4CW9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aromatase (Homo sapiens (Human)) | BDBM9460 (3-(4-aminophenyl)-3-ethyl-piperidine-2,6-dione | 3...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank KEGG PC cid PC sid PDB UniChem Patents Similars | DrugBank PubMed | n/a | n/a | 1.07E+6 | n/a | n/a | n/a | n/a | n/a | n/a |
Johns Hopkins University School of Medicine Curated by ChEMBL | Assay Description Inhibition of Cytochrome P450 19A1 activity 6 hr after oral administration | J Med Chem 33: 2933-42 (1990) BindingDB Entry DOI: 10.7270/Q2VM4CW9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||