

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| ALK tyrosine kinase receptor (Homo sapiens (Human)) | BDBM50240271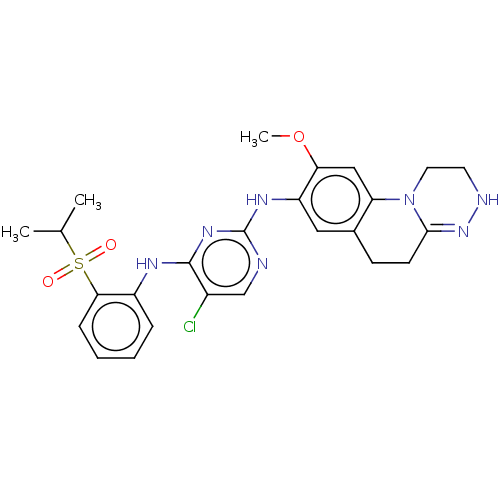 (CHEMBL4101954) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.330 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Science& Technology Curated by ChEMBL | Assay Description In vitro ability to inhibit the binding of [3H]spiperone to dopamine receptor D2 in rat striatal membranes. | Bioorg Med Chem Lett 27: 2185-2191 (2017) Article DOI: 10.1016/j.bmcl.2017.03.073 BindingDB Entry DOI: 10.7270/Q2DR2XNC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| ALK tyrosine kinase receptor (Homo sapiens (Human)) | BDBM50240270 (CHEMBL4099922) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | 1.70 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Science& Technology Curated by ChEMBL | Assay Description In vitro ability to inhibit the binding of [3H]spiperone to dopamine receptor D2 in rat striatal membranes. | Bioorg Med Chem Lett 27: 2185-2191 (2017) Article DOI: 10.1016/j.bmcl.2017.03.073 BindingDB Entry DOI: 10.7270/Q2DR2XNC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| ALK tyrosine kinase receptor (Homo sapiens (Human)) | BDBM50240274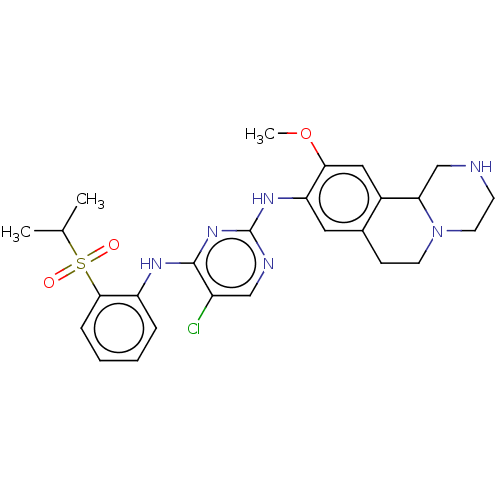 (CHEMBL4092174) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Science& Technology Curated by ChEMBL | Assay Description Inhibition of wild type ALK (unknown origin) using TK as substrate after 30 mins by HTRF assay | Bioorg Med Chem Lett 27: 2185-2191 (2017) Article DOI: 10.1016/j.bmcl.2017.03.073 BindingDB Entry DOI: 10.7270/Q2DR2XNC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Insulin receptor (Homo sapiens (Human)) | BDBM50240274 (CHEMBL4092174) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2.10 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Science& Technology Curated by ChEMBL | Assay Description In vitro ability to inhibit the binding of [3H]spiperone to dopamine receptor D2 in rat striatal membranes. | Bioorg Med Chem Lett 27: 2185-2191 (2017) Article DOI: 10.1016/j.bmcl.2017.03.073 BindingDB Entry DOI: 10.7270/Q2DR2XNC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Insulin receptor (Homo sapiens (Human)) | BDBM244039 (10-((5-chloro-4-(2-(isopropylsulfonyl)phenyl)amino...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 2.20 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Science& Technology Curated by ChEMBL | Assay Description In vitro ability to inhibit the binding of [3H]spiperone to dopamine receptor D2 in rat striatal membranes. | Bioorg Med Chem Lett 27: 2185-2191 (2017) Article DOI: 10.1016/j.bmcl.2017.03.073 BindingDB Entry DOI: 10.7270/Q2DR2XNC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Insulin receptor (Homo sapiens (Human)) | BDBM244025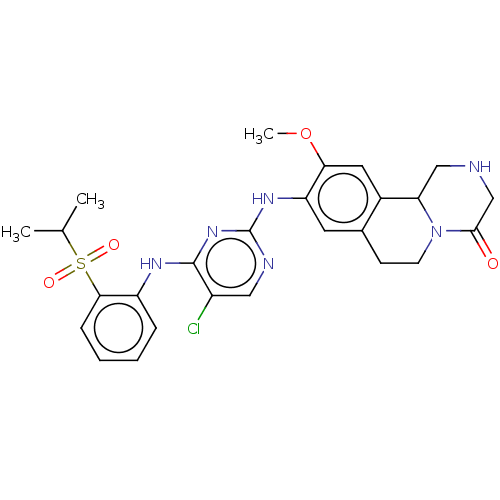 (9-((5-chloro-4-(2-(isopropylsulfonyl)phenyl)aminop...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 2.80 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Science& Technology Curated by ChEMBL | Assay Description Inhibition of insulin receptor kinase (unknown origin) using TK as substrate after 30 mins by HTRF assay | Bioorg Med Chem Lett 27: 2185-2191 (2017) Article DOI: 10.1016/j.bmcl.2017.03.073 BindingDB Entry DOI: 10.7270/Q2DR2XNC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| ALK tyrosine kinase receptor (Homo sapiens (Human)) | BDBM244025 (9-((5-chloro-4-(2-(isopropylsulfonyl)phenyl)aminop...) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 3.70 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Science& Technology Curated by ChEMBL | Assay Description Inhibition of wild type ALK (unknown origin) using TK as substrate after 30 mins by HTRF assay | Bioorg Med Chem Lett 27: 2185-2191 (2017) Article DOI: 10.1016/j.bmcl.2017.03.073 BindingDB Entry DOI: 10.7270/Q2DR2XNC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| ALK tyrosine kinase receptor (Homo sapiens (Human)) | BDBM244039 (10-((5-chloro-4-(2-(isopropylsulfonyl)phenyl)amino...) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 4.30 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Science& Technology Curated by ChEMBL | Assay Description Inhibition of wild type ALK (unknown origin) using TK as substrate after 30 mins by HTRF assay | Bioorg Med Chem Lett 27: 2185-2191 (2017) Article DOI: 10.1016/j.bmcl.2017.03.073 BindingDB Entry DOI: 10.7270/Q2DR2XNC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| ALK tyrosine kinase receptor (Homo sapiens (Human)) | BDBM50240276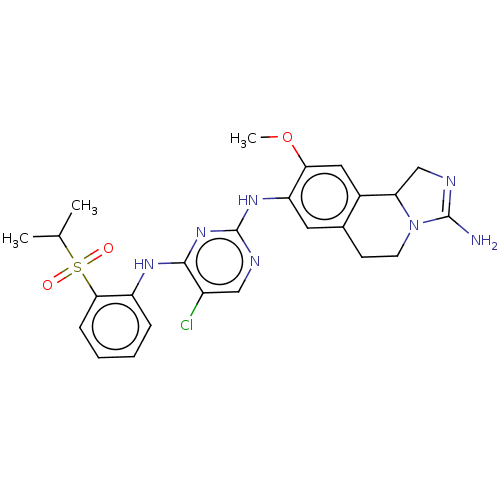 (CHEMBL4083964) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 5.30 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Science& Technology Curated by ChEMBL | Assay Description Inhibition of wild type ALK (unknown origin) using TK as substrate after 30 mins by HTRF assay | Bioorg Med Chem Lett 27: 2185-2191 (2017) Article DOI: 10.1016/j.bmcl.2017.03.073 BindingDB Entry DOI: 10.7270/Q2DR2XNC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Insulin receptor (Homo sapiens (Human)) | BDBM50240270 (CHEMBL4099922) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | 6 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Science& Technology Curated by ChEMBL | Assay Description Inhibition of insulin receptor kinase (unknown origin) using TK as substrate after 30 mins by HTRF assay | Bioorg Med Chem Lett 27: 2185-2191 (2017) Article DOI: 10.1016/j.bmcl.2017.03.073 BindingDB Entry DOI: 10.7270/Q2DR2XNC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Insulin receptor (Homo sapiens (Human)) | BDBM50240272 (CHEMBL4063965) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 7.5 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Science& Technology Curated by ChEMBL | Assay Description Inhibition of insulin receptor kinase (unknown origin) using TK as substrate after 30 mins by HTRF assay | Bioorg Med Chem Lett 27: 2185-2191 (2017) Article DOI: 10.1016/j.bmcl.2017.03.073 BindingDB Entry DOI: 10.7270/Q2DR2XNC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Insulin receptor (Homo sapiens (Human)) | BDBM50240271 (CHEMBL4101954) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 11 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Science& Technology Curated by ChEMBL | Assay Description Inhibition of insulin receptor kinase (unknown origin) using TK as substrate after 30 mins by HTRF assay | Bioorg Med Chem Lett 27: 2185-2191 (2017) Article DOI: 10.1016/j.bmcl.2017.03.073 BindingDB Entry DOI: 10.7270/Q2DR2XNC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| ALK tyrosine kinase receptor (Homo sapiens (Human)) | BDBM244040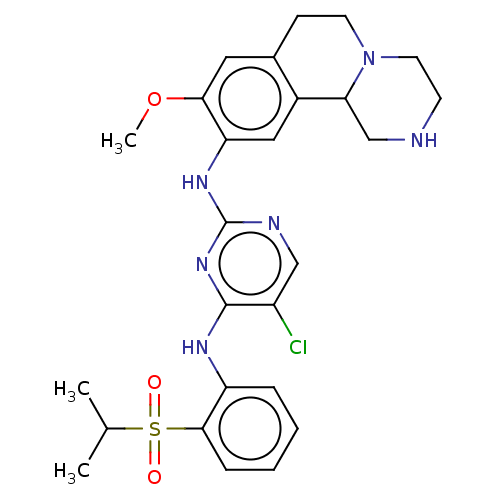 (5-chloro-N4-(2-(isopropylsulfonyl)phenyl)-N2-(9-me...) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 12 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Science& Technology Curated by ChEMBL | Assay Description Inhibition of wild type ALK (unknown origin) using TK as substrate after 30 mins by HTRF assay | Bioorg Med Chem Lett 27: 2185-2191 (2017) Article DOI: 10.1016/j.bmcl.2017.03.073 BindingDB Entry DOI: 10.7270/Q2DR2XNC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Insulin receptor (Homo sapiens (Human)) | BDBM244040 (5-chloro-N4-(2-(isopropylsulfonyl)phenyl)-N2-(9-me...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 14 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Science& Technology Curated by ChEMBL | Assay Description Inhibition of insulin receptor kinase (unknown origin) using TK as substrate after 30 mins by HTRF assay | Bioorg Med Chem Lett 27: 2185-2191 (2017) Article DOI: 10.1016/j.bmcl.2017.03.073 BindingDB Entry DOI: 10.7270/Q2DR2XNC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| ALK tyrosine kinase receptor (Homo sapiens (Human)) | BDBM50436850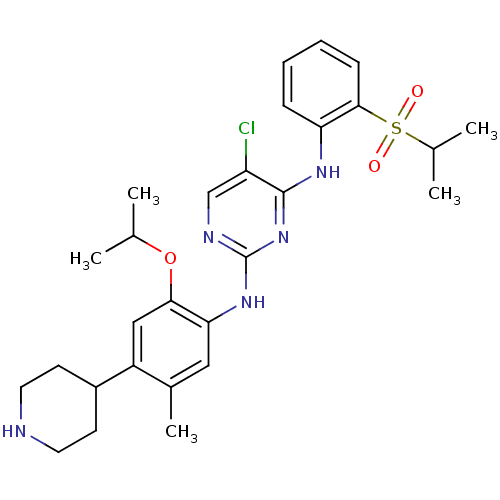 (CERITINIB | CHEMBL2403108 | LDK378 | US10053458, C...) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE MMDB PC cid PC sid PDB UniChem Similars | PDB Article PubMed | n/a | n/a | 14 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Science& Technology Curated by ChEMBL | Assay Description Inhibition of wild type ALK (unknown origin) using TK as substrate after 30 mins by HTRF assay | Bioorg Med Chem Lett 27: 2185-2191 (2017) Article DOI: 10.1016/j.bmcl.2017.03.073 BindingDB Entry DOI: 10.7270/Q2DR2XNC | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| ALK tyrosine kinase receptor (Homo sapiens (Human)) | BDBM50240272 (CHEMBL4063965) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 21 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Science& Technology Curated by ChEMBL | Assay Description In vitro ability to inhibit the binding of [3H]spiperone to dopamine receptor D2 in rat striatal membranes. | Bioorg Med Chem Lett 27: 2185-2191 (2017) Article DOI: 10.1016/j.bmcl.2017.03.073 BindingDB Entry DOI: 10.7270/Q2DR2XNC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Insulin receptor (Homo sapiens (Human)) | BDBM50240276 (CHEMBL4083964) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 21 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Science& Technology Curated by ChEMBL | Assay Description Inhibition of insulin receptor kinase (unknown origin) using TK as substrate after 30 mins by HTRF assay | Bioorg Med Chem Lett 27: 2185-2191 (2017) Article DOI: 10.1016/j.bmcl.2017.03.073 BindingDB Entry DOI: 10.7270/Q2DR2XNC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Insulin receptor (Homo sapiens (Human)) | BDBM50436850 (CERITINIB | CHEMBL2403108 | LDK378 | US10053458, C...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE MMDB PC cid PC sid PDB UniChem Similars | Article PubMed | n/a | n/a | 24 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Science& Technology Curated by ChEMBL | Assay Description Inhibition of insulin receptor kinase (unknown origin) using TK as substrate after 30 mins by HTRF assay | Bioorg Med Chem Lett 27: 2185-2191 (2017) Article DOI: 10.1016/j.bmcl.2017.03.073 BindingDB Entry DOI: 10.7270/Q2DR2XNC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| ALK tyrosine kinase receptor (Homo sapiens (Human)) | BDBM50240273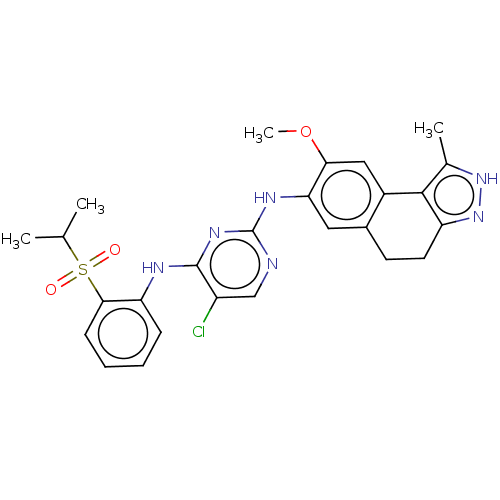 (CHEMBL4091729) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 29 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Science& Technology Curated by ChEMBL | Assay Description In vitro ability to inhibit the binding of [3H]spiperone to dopamine receptor D2 in rat striatal membranes. | Bioorg Med Chem Lett 27: 2185-2191 (2017) Article DOI: 10.1016/j.bmcl.2017.03.073 BindingDB Entry DOI: 10.7270/Q2DR2XNC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Insulin receptor (Homo sapiens (Human)) | BDBM50240273 (CHEMBL4091729) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 130 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Science& Technology Curated by ChEMBL | Assay Description Inhibition of insulin receptor kinase (unknown origin) using TK as substrate after 30 mins by HTRF assay | Bioorg Med Chem Lett 27: 2185-2191 (2017) Article DOI: 10.1016/j.bmcl.2017.03.073 BindingDB Entry DOI: 10.7270/Q2DR2XNC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| ALK tyrosine kinase receptor (Homo sapiens (Human)) | BDBM50240269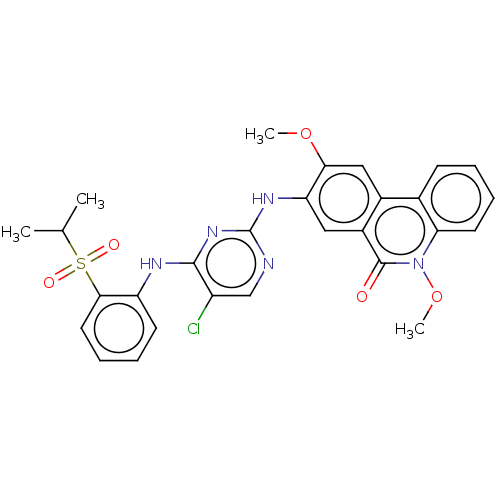 (CHEMBL4062738) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 190 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Science& Technology Curated by ChEMBL | Assay Description Inhibition of [3H]spiperone binding to rat striatal membrane Dopamine receptor D2 | Bioorg Med Chem Lett 27: 2185-2191 (2017) Article DOI: 10.1016/j.bmcl.2017.03.073 BindingDB Entry DOI: 10.7270/Q2DR2XNC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Insulin receptor (Homo sapiens (Human)) | BDBM50240275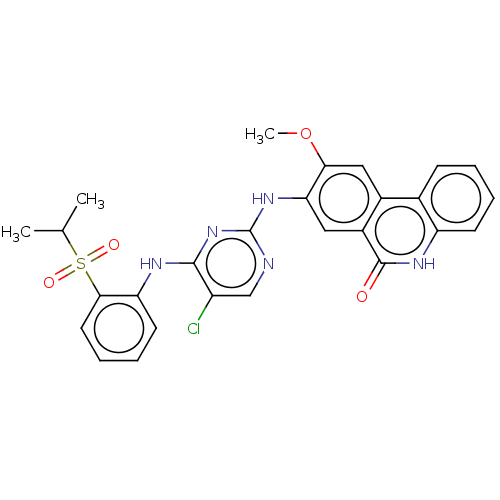 (CHEMBL4074115) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 260 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Science& Technology Curated by ChEMBL | Assay Description Inhibition of insulin receptor kinase (unknown origin) using TK as substrate after 30 mins by HTRF assay | Bioorg Med Chem Lett 27: 2185-2191 (2017) Article DOI: 10.1016/j.bmcl.2017.03.073 BindingDB Entry DOI: 10.7270/Q2DR2XNC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Insulin receptor (Homo sapiens (Human)) | BDBM50240269 (CHEMBL4062738) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 300 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Science& Technology Curated by ChEMBL | Assay Description Inhibition of insulin receptor kinase (unknown origin) using TK as substrate after 30 mins by HTRF assay | Bioorg Med Chem Lett 27: 2185-2191 (2017) Article DOI: 10.1016/j.bmcl.2017.03.073 BindingDB Entry DOI: 10.7270/Q2DR2XNC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| ALK tyrosine kinase receptor (Homo sapiens (Human)) | BDBM50240275 (CHEMBL4074115) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 310 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Science& Technology Curated by ChEMBL | Assay Description Inhibition of wild type ALK (unknown origin) using TK as substrate after 30 mins by HTRF assay | Bioorg Med Chem Lett 27: 2185-2191 (2017) Article DOI: 10.1016/j.bmcl.2017.03.073 BindingDB Entry DOI: 10.7270/Q2DR2XNC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| ALK tyrosine kinase receptor (Homo sapiens (Human)) | BDBM50240268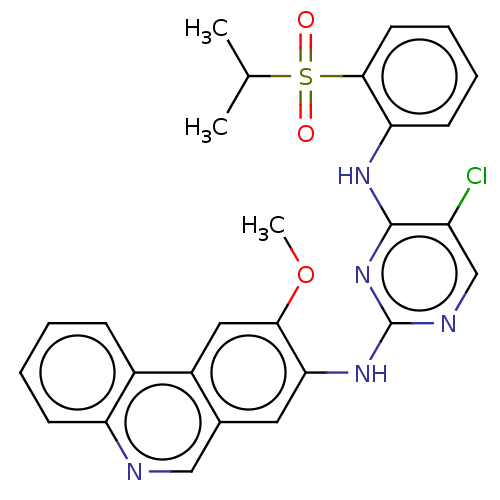 (CHEMBL4100875) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Science& Technology Curated by ChEMBL | Assay Description Inhibition of wild type ALK (unknown origin) using TK as substrate after 30 mins by HTRF assay | Bioorg Med Chem Lett 27: 2185-2191 (2017) Article DOI: 10.1016/j.bmcl.2017.03.073 BindingDB Entry DOI: 10.7270/Q2DR2XNC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Insulin receptor (Homo sapiens (Human)) | BDBM50240268 (CHEMBL4100875) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Science& Technology Curated by ChEMBL | Assay Description In vitro ability to inhibit the binding of [3H]spiperone to dopamine receptor D2 in rat striatal membranes. | Bioorg Med Chem Lett 27: 2185-2191 (2017) Article DOI: 10.1016/j.bmcl.2017.03.073 BindingDB Entry DOI: 10.7270/Q2DR2XNC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||