

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Neuronal acetylcholine receptor subunit alpha-4/beta-2 (Homo sapiens (Human)) | BDBM50049750 ((S)-3-(azetidin-2-ylmethoxy)pyridine | 3-((S)-1-Az...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | Article PubMed | 0.0400 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratory Curated by ChEMBL | Assay Description Compound was evaluated for its ability to displace [3H]-cytisine binding from high-affinity Nicotinic acetylcholine receptor in rat brain (principall... | J Med Chem 41: 407-12 (1998) Article DOI: 10.1021/jm9706224 BindingDB Entry DOI: 10.7270/Q2CJ8F56 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuronal acetylcholine receptor subunit alpha-4/beta-2 (Homo sapiens (Human)) | BDBM50062641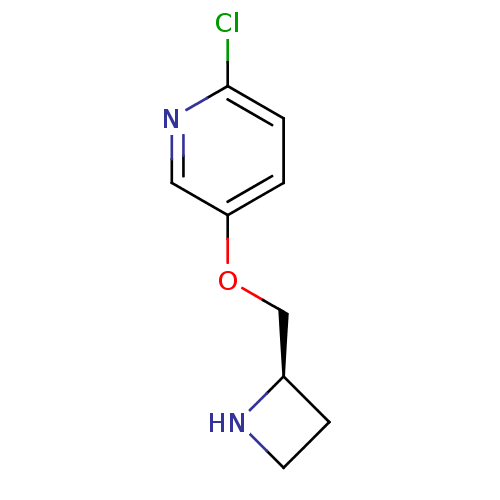 (5-((R)-1-Azetidin-2-ylmethoxy)-2-chloro-pyridine |...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | Article PubMed | 0.0550 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratory Curated by ChEMBL | Assay Description Compound was evaluated for its ability to displace [125I]alpha-bungarotoxin (alpha-BgT) from K28 cells expressing human Nicotinic acetylcholine recep... | J Med Chem 41: 407-12 (1998) Article DOI: 10.1021/jm9706224 BindingDB Entry DOI: 10.7270/Q2CJ8F56 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuronal acetylcholine receptor subunit alpha-4/beta-2 (Homo sapiens (Human)) | BDBM50062641 (5-((R)-1-Azetidin-2-ylmethoxy)-2-chloro-pyridine |...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | Article PubMed | 0.0550 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratory Curated by ChEMBL | Assay Description Compound was evaluated for its ability to displace [3H]cytisine from K177 cells expressing human Nicotinic acetylcholine receptor alpha4-beta2 | J Med Chem 41: 407-12 (1998) Article DOI: 10.1021/jm9706224 BindingDB Entry DOI: 10.7270/Q2CJ8F56 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuronal acetylcholine receptor subunit alpha-7 (Homo sapiens (Human)) | BDBM50049750 ((S)-3-(azetidin-2-ylmethoxy)pyridine | 3-((S)-1-Az...) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | Article PubMed | 148 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratory Curated by ChEMBL | Assay Description Compound was evaluated for its ability to displace [125I]alpha-bungarotoxin (alpha-BgT) from K28 cells expressing human Nicotinic acetylcholine recep... | J Med Chem 41: 407-12 (1998) Article DOI: 10.1021/jm9706224 BindingDB Entry DOI: 10.7270/Q2CJ8F56 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholine receptor subunit alpha (Torpedo californica) | BDBM50049750 ((S)-3-(azetidin-2-ylmethoxy)pyridine | 3-((S)-1-Az...) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | Article PubMed | 314 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratory Curated by ChEMBL | Assay Description Compound was evaluated for its ability to displace [125 I]alpha-bungarotoxin (alpha-BgT) from torpedo alpha1-beta1-gamma1 electroplax | J Med Chem 41: 407-12 (1998) Article DOI: 10.1021/jm9706224 BindingDB Entry DOI: 10.7270/Q2CJ8F56 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholine receptor subunit alpha (Torpedo californica) | BDBM50049753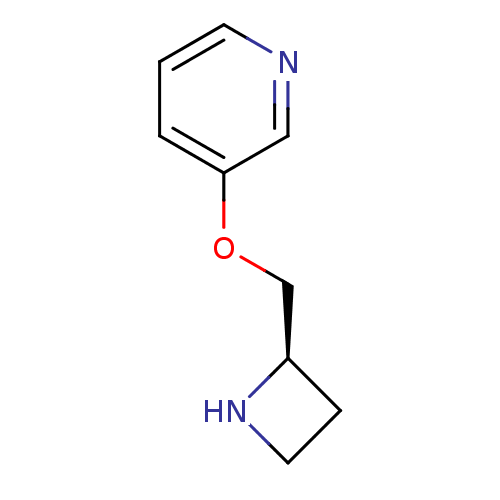 (3-((R)-1-Azetidin-2-ylmethoxy)-pyridine | 3-((S)-1...) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 384 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratory Curated by ChEMBL | Assay Description Compound was evaluated for its ability to displace [125 I]alpha-bungarotoxin (alpha-BgT) from torpedo alpha1-beta1-gamma1 electroplax | J Med Chem 41: 407-12 (1998) Article DOI: 10.1021/jm9706224 BindingDB Entry DOI: 10.7270/Q2CJ8F56 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuronal acetylcholine receptor subunit alpha-7 (Homo sapiens (Human)) | BDBM50049753 (3-((R)-1-Azetidin-2-ylmethoxy)-pyridine | 3-((S)-1...) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1.28E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratory Curated by ChEMBL | Assay Description Compound was evaluated for its ability to displace [125I]alpha-bungarotoxin (alpha-BgT) from K28 cells expressing human Nicotinic acetylcholine recep... | J Med Chem 41: 407-12 (1998) Article DOI: 10.1021/jm9706224 BindingDB Entry DOI: 10.7270/Q2CJ8F56 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuronal acetylcholine receptor subunit alpha-7 (Homo sapiens (Human)) | BDBM50062641 (5-((R)-1-Azetidin-2-ylmethoxy)-2-chloro-pyridine |...) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | Article PubMed | 2.06E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratory Curated by ChEMBL | Assay Description Compound was evaluated for functional activation from channel currents at human Nicotinic acetylcholine receptor alpha-7 expressed in oocytes. | J Med Chem 41: 407-12 (1998) Article DOI: 10.1021/jm9706224 BindingDB Entry DOI: 10.7270/Q2CJ8F56 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuronal acetylcholine receptor subunit alpha-7 (Homo sapiens (Human)) | BDBM50062641 (5-((R)-1-Azetidin-2-ylmethoxy)-2-chloro-pyridine |...) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | Article PubMed | 2.06E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratory Curated by ChEMBL | Assay Description Functional activation of human sympathetic ganglionic type nAChRs in IMR-32 cells containing alpha-3 subtype | J Med Chem 41: 407-12 (1998) Article DOI: 10.1021/jm9706224 BindingDB Entry DOI: 10.7270/Q2CJ8F56 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholine receptor subunit alpha (Torpedo californica) | BDBM50062641 (5-((R)-1-Azetidin-2-ylmethoxy)-2-chloro-pyridine |...) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | Article PubMed | 1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratory Curated by ChEMBL | Assay Description Compound was evaluated for its ability to displace [125 I]alpha-bungarotoxin (alpha-BgT) from torpedo alpha1-beta1-gamma1 electroplax | J Med Chem 41: 407-12 (1998) Article DOI: 10.1021/jm9706224 BindingDB Entry DOI: 10.7270/Q2CJ8F56 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholine receptor subunit alpha (Torpedo californica) | BDBM50062641 (5-((R)-1-Azetidin-2-ylmethoxy)-2-chloro-pyridine |...) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | Article PubMed | 1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratory Curated by ChEMBL | Assay Description Binding affinity against Nicotinic Acetylcholine Receptor was determined by measuring the displacement of [3H]-cytisine from a preparation of whole r... | J Med Chem 41: 407-12 (1998) Article DOI: 10.1021/jm9706224 BindingDB Entry DOI: 10.7270/Q2CJ8F56 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuronal acetylcholine receptor subunit alpha-3 (Homo sapiens (Human)) | BDBM50062642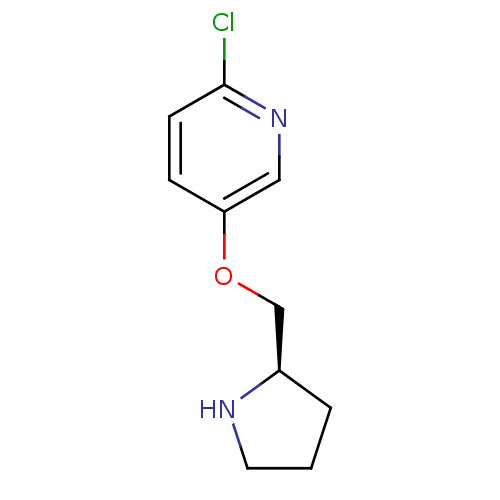 (2-Chloro-5-((R)-1-pyrrolidin-2-ylmethoxy)-pyridine...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 500 | n/a | n/a | n/a | n/a |
Abbott Laboratory Curated by ChEMBL | Assay Description Functional activation of human sympathetic ganglionic type nAChRs in IMR-32 cells containing alpha-3 subtype | J Med Chem 41: 407-12 (1998) Article DOI: 10.1021/jm9706224 BindingDB Entry DOI: 10.7270/Q2CJ8F56 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuronal acetylcholine receptor subunit alpha-3 (Homo sapiens (Human)) | BDBM50062643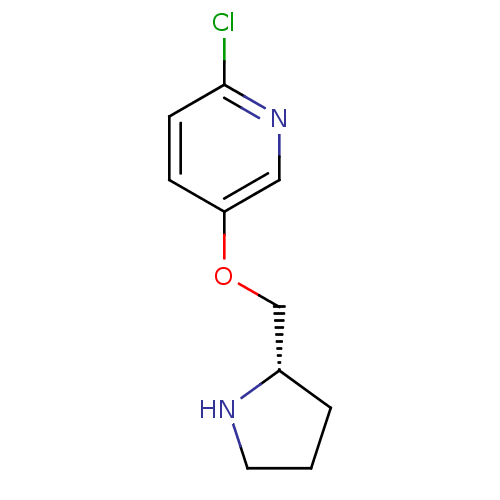 (2-Chloro-5-((S)-1-pyrrolidin-2-ylmethoxy)-pyridine...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | n/a | n/a | 1.19E+3 | n/a | n/a | n/a | n/a |
Abbott Laboratory Curated by ChEMBL | Assay Description Functional activation of human sympathetic ganglionic type nAChRs in IMR-32 cells containing alpha-3 subtype | J Med Chem 41: 407-12 (1998) Article DOI: 10.1021/jm9706224 BindingDB Entry DOI: 10.7270/Q2CJ8F56 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuronal acetylcholine receptor subunit alpha-3 (Homo sapiens (Human)) | BDBM50049757 (()-2-(6-Chloro-pyridin-3-yl)-7-aza-bicyclo[2.2.1]h...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | n/a | n/a | 7 | n/a | n/a | n/a | n/a |
Abbott Laboratory Curated by ChEMBL | Assay Description Functional activation of human sympathetic ganglionic type nAChRs in IMR-32 cells containing alpha-3 subtype | J Med Chem 41: 407-12 (1998) Article DOI: 10.1021/jm9706224 BindingDB Entry DOI: 10.7270/Q2CJ8F56 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuronal acetylcholine receptor subunit alpha-3 (Homo sapiens (Human)) | BDBM50049750 ((S)-3-(azetidin-2-ylmethoxy)pyridine | 3-((S)-1-Az...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | n/a | n/a | 700 | n/a | n/a | n/a | n/a |
Abbott Laboratory Curated by ChEMBL | Assay Description Functional activation of human sympathetic ganglionic type nAChRs in IMR-32 cells containing alpha-3 subtype | J Med Chem 41: 407-12 (1998) Article DOI: 10.1021/jm9706224 BindingDB Entry DOI: 10.7270/Q2CJ8F56 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuronal acetylcholine receptor subunit alpha-3 (Homo sapiens (Human)) | BDBM50062641 (5-((R)-1-Azetidin-2-ylmethoxy)-2-chloro-pyridine |...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | n/a | n/a | 200 | n/a | n/a | n/a | n/a |
Abbott Laboratory Curated by ChEMBL | Assay Description Functional activation of human sympathetic ganglionic type nAChRs in IMR-32 cells containing alpha-3 subtype | J Med Chem 41: 407-12 (1998) Article DOI: 10.1021/jm9706224 BindingDB Entry DOI: 10.7270/Q2CJ8F56 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuronal acetylcholine receptor subunit alpha-3 (Homo sapiens (Human)) | BDBM50062640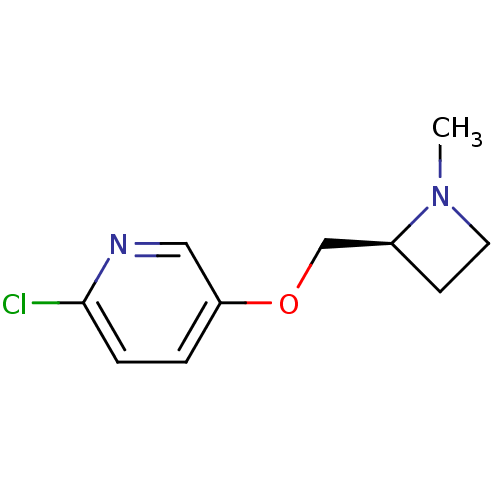 (2-Chloro-5-((S)-1-methyl-azetidin-2-ylmethoxy)-pyr...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 2.89E+4 | n/a | n/a | n/a | n/a |
Abbott Laboratory Curated by ChEMBL | Assay Description Functional activation of human sympathetic ganglionic type nAChRs in IMR-32 cells containing alpha-3 subtype | J Med Chem 41: 407-12 (1998) Article DOI: 10.1021/jm9706224 BindingDB Entry DOI: 10.7270/Q2CJ8F56 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuronal acetylcholine receptor subunit alpha-3 (Homo sapiens (Human)) | BDBM50062641 (5-((R)-1-Azetidin-2-ylmethoxy)-2-chloro-pyridine |...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | n/a | n/a | 200 | n/a | n/a | n/a | n/a |
Abbott Laboratory Curated by ChEMBL | Assay Description Bindind affinity value obtained by measuring the displacement of radioligand [3H]-(-)-cytisine from whole rat brain Nicotinic acetylcholine receptor | J Med Chem 41: 407-12 (1998) Article DOI: 10.1021/jm9706224 BindingDB Entry DOI: 10.7270/Q2CJ8F56 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuronal acetylcholine receptor subunit alpha-7 (Homo sapiens (Human)) | BDBM50049753 (3-((R)-1-Azetidin-2-ylmethoxy)-pyridine | 3-((S)-1...) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 3.60E+4 | n/a | n/a | n/a | n/a |
Abbott Laboratory Curated by ChEMBL | Assay Description Compound was evaluated for its ability to displace [3H]cytisine from K177 cells expressing human Nicotinic acetylcholine receptor alpha4-beta2 | J Med Chem 41: 407-12 (1998) Article DOI: 10.1021/jm9706224 BindingDB Entry DOI: 10.7270/Q2CJ8F56 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuronal acetylcholine receptor subunit alpha-7 (Homo sapiens (Human)) | BDBM50062641 (5-((R)-1-Azetidin-2-ylmethoxy)-2-chloro-pyridine |...) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | n/a | n/a | 2.10E+4 | n/a | n/a | n/a | n/a |
Abbott Laboratory Curated by ChEMBL | Assay Description Compound was evaluated for functional activation from channel currents at human Nicotinic acetylcholine receptor alpha-7 expressed in oocytes. | J Med Chem 41: 407-12 (1998) Article DOI: 10.1021/jm9706224 BindingDB Entry DOI: 10.7270/Q2CJ8F56 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuronal acetylcholine receptor subunit alpha-7 (Homo sapiens (Human)) | BDBM50062641 (5-((R)-1-Azetidin-2-ylmethoxy)-2-chloro-pyridine |...) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | n/a | n/a | 5.60E+4 | n/a | n/a | n/a | n/a |
Abbott Laboratory Curated by ChEMBL | Assay Description Compound was evaluated for functional activation from channel currents at human Nicotinic acetylcholine receptor alpha-7 expressed in oocytes. | J Med Chem 41: 407-12 (1998) Article DOI: 10.1021/jm9706224 BindingDB Entry DOI: 10.7270/Q2CJ8F56 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuronal acetylcholine receptor subunit alpha-3 (Homo sapiens (Human)) | BDBM50049753 (3-((R)-1-Azetidin-2-ylmethoxy)-pyridine | 3-((S)-1...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 1.10E+3 | n/a | n/a | n/a | n/a |
Abbott Laboratory Curated by ChEMBL | Assay Description Functional activation of human sympathetic ganglionic type nAChRs in IMR-32 cells containing alpha-3 subtype | J Med Chem 41: 407-12 (1998) Article DOI: 10.1021/jm9706224 BindingDB Entry DOI: 10.7270/Q2CJ8F56 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuronal acetylcholine receptor subunit alpha-7 (Homo sapiens (Human)) | BDBM50049750 ((S)-3-(azetidin-2-ylmethoxy)pyridine | 3-((S)-1-Az...) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | n/a | n/a | 8.90E+3 | n/a | n/a | n/a | n/a |
Abbott Laboratory Curated by ChEMBL | Assay Description Binding affinity against Nicotinic Acetylcholine Receptor was determined by measuring the displacement of [3H]-cytisine from a preparation of whole r... | J Med Chem 41: 407-12 (1998) Article DOI: 10.1021/jm9706224 BindingDB Entry DOI: 10.7270/Q2CJ8F56 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||