

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Carbonic anhydrase 2 (Homo sapiens (Human)) | BDBM50079040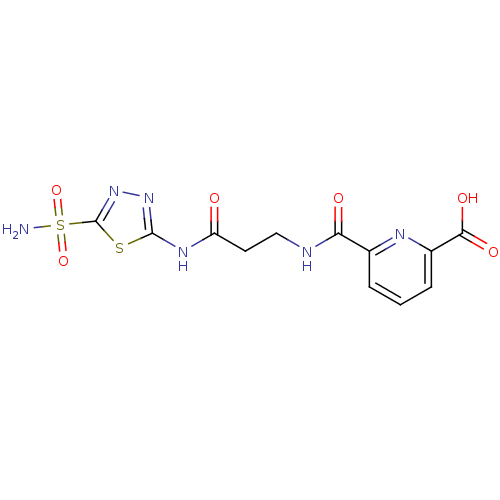 (6-[2-(5-Sulfamoyl-[1,3,4]thiadiazol-2-ylcarbamoyl)...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 2 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ degli Studi Curated by ChEMBL | Assay Description Inhibitory activity against human recombinant carbonic anhydrase II | J Med Chem 42: 2641-50 (1999) Article DOI: 10.1021/jm9900523 BindingDB Entry DOI: 10.7270/Q2J67HMK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carbonic anhydrase 2 (Homo sapiens (Human)) | BDBM50079059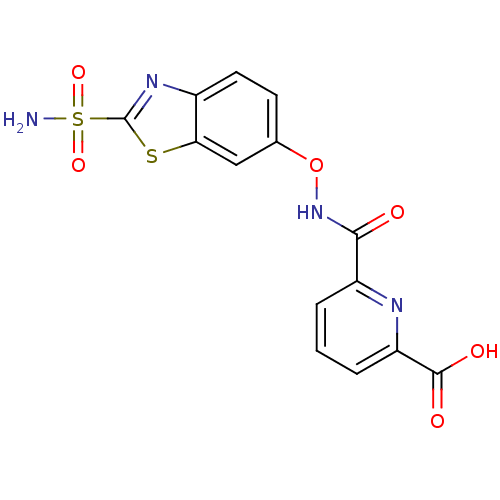 (6-(2-Sulfamoyl-benzothiazol-6-yloxycarbamoyl)-pyri...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 2 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ degli Studi Curated by ChEMBL | Assay Description Inhibitory activity against human recombinant carbonic anhydrase II | J Med Chem 42: 2641-50 (1999) Article DOI: 10.1021/jm9900523 BindingDB Entry DOI: 10.7270/Q2J67HMK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carbonic anhydrase 2 (Homo sapiens (Human)) | BDBM50079068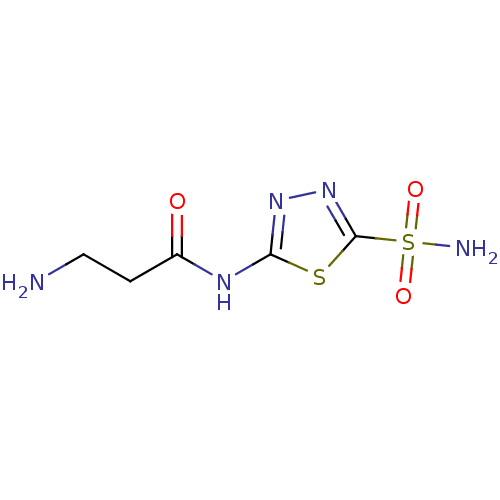 (3-amino-N-(5-sulfamoyl-[1,3,4]thiadiazol-2-yl)-pro...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ degli Studi Curated by ChEMBL | Assay Description Inhibitory activity against human cloned Carbonic anhydrase II (hCA II,cytosolic form). | J Med Chem 42: 2641-50 (1999) Article DOI: 10.1021/jm9900523 BindingDB Entry DOI: 10.7270/Q2J67HMK | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (docked) | ||||||||||||
| Carbonic anhydrase 2 (Homo sapiens (Human)) | BDBM50079034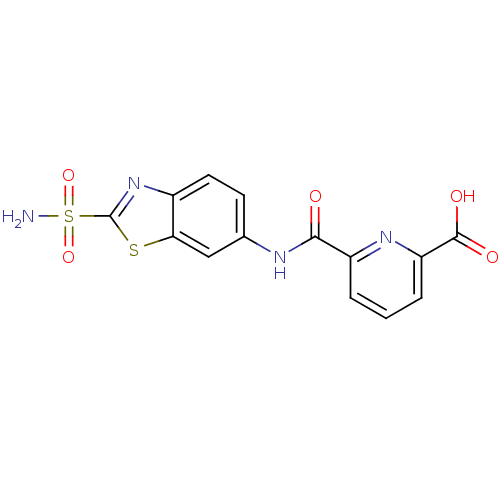 (6-(2-Sulfamoyl-benzothiazol-6-ylcarbamoyl)-pyridin...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | 3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ degli Studi Curated by ChEMBL | Assay Description Inhibitory activity against human recombinant carbonic anhydrase II | J Med Chem 42: 2641-50 (1999) Article DOI: 10.1021/jm9900523 BindingDB Entry DOI: 10.7270/Q2J67HMK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carbonic anhydrase 2 (Homo sapiens (Human)) | BDBM50079062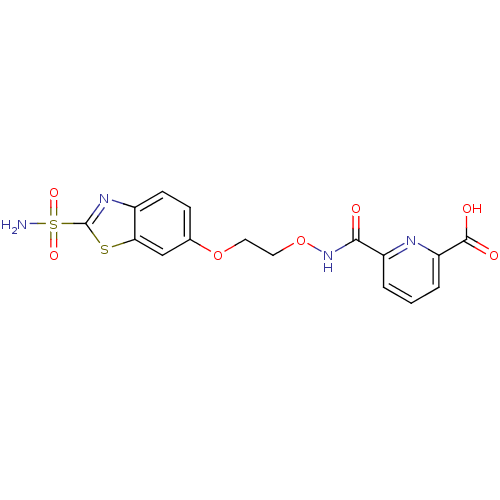 (6-[2-(2-Sulfamoyl-benzothiazol-6-yloxy)-ethoxycarb...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ degli Studi Curated by ChEMBL | Assay Description Inhibitory activity against human recombinant carbonic anhydrase II | J Med Chem 42: 2641-50 (1999) Article DOI: 10.1021/jm9900523 BindingDB Entry DOI: 10.7270/Q2J67HMK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carbonic anhydrase 2 (Homo sapiens (Human)) | BDBM50079042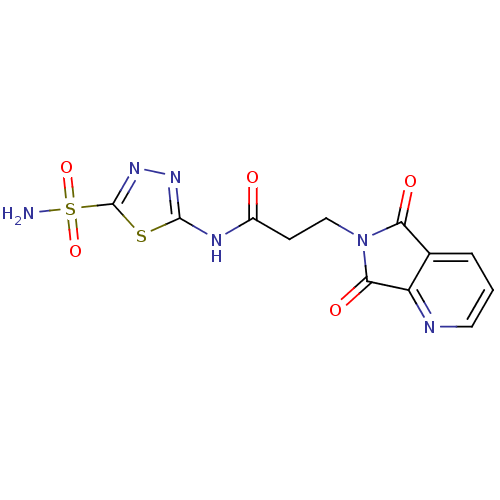 (3-(5,7-Dioxo-5,7-dihydro-pyrrolo[3,4-b]pyridin-6-y...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ degli Studi Curated by ChEMBL | Assay Description Inhibitory activity against human cloned Carbonic anhydrase II (hCA II,cytosolic form). | J Med Chem 42: 2641-50 (1999) Article DOI: 10.1021/jm9900523 BindingDB Entry DOI: 10.7270/Q2J67HMK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carbonic anhydrase 2 (Homo sapiens (Human)) | BDBM10885 ((4R)-4-(ethylamino)-2-(3-methoxypropyl)-1,1-dioxo-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | DrugBank Article PubMed | 3.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ degli Studi Curated by ChEMBL | Assay Description Inhibitory activity against human recombinant carbonic anhydrase II | J Med Chem 42: 2641-50 (1999) Article DOI: 10.1021/jm9900523 BindingDB Entry DOI: 10.7270/Q2J67HMK | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (docked) | ||||||||||||
| Carbonic anhydrase 2 (Homo sapiens (Human)) | BDBM50079031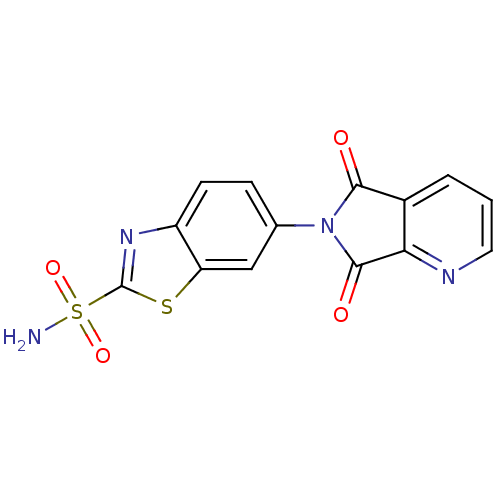 (6-(5,7-Dioxo-5,7-dihydro-pyrrolo[3,4-b]pyridin-6-y...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | 4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ degli Studi Curated by ChEMBL | Assay Description Inhibitory activity against human cloned Carbonic anhydrase II (hCA II,cytosolic form). | J Med Chem 42: 2641-50 (1999) Article DOI: 10.1021/jm9900523 BindingDB Entry DOI: 10.7270/Q2J67HMK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carbonic anhydrase 2 (Homo sapiens (Human)) | BDBM50079048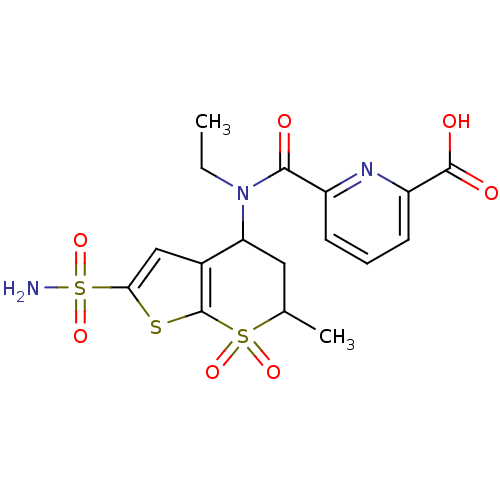 (6-[Ethyl-(6-methyl-7,7-dioxo-2-sulfamoyl-4,5,6,7-t...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ degli Studi Curated by ChEMBL | Assay Description Inhibitory activity against human recombinant carbonic anhydrase II | J Med Chem 42: 2641-50 (1999) Article DOI: 10.1021/jm9900523 BindingDB Entry DOI: 10.7270/Q2J67HMK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carbonic anhydrase 2 (Homo sapiens (Human)) | BDBM50079056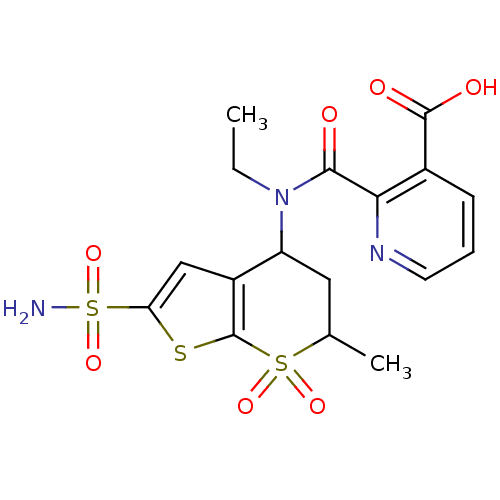 (2-[Ethyl-(6-methyl-7,7-dioxo-2-sulfamoyl-4,5,6,7-t...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ degli Studi Curated by ChEMBL | Assay Description Inhibitory activity against human recombinant carbonic anhydrase II | J Med Chem 42: 2641-50 (1999) Article DOI: 10.1021/jm9900523 BindingDB Entry DOI: 10.7270/Q2J67HMK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carbonic anhydrase 2 (Homo sapiens (Human)) | BDBM50079037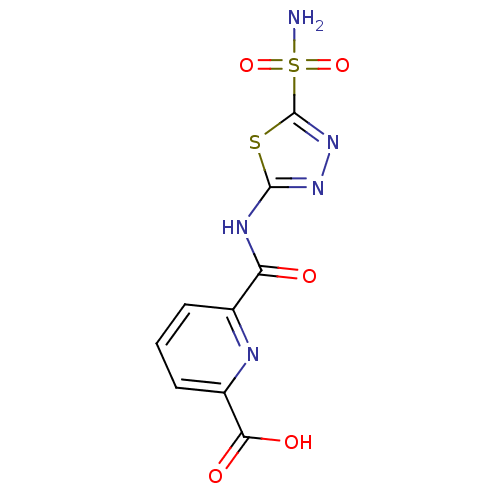 (6-(5-Sulfamoyl-[1,3,4]thiadiazol-2-ylcarbamoyl)-py...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 6 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ degli Studi Curated by ChEMBL | Assay Description Inhibitory activity against human recombinant carbonic anhydrase II | J Med Chem 42: 2641-50 (1999) Article DOI: 10.1021/jm9900523 BindingDB Entry DOI: 10.7270/Q2J67HMK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carbonic anhydrase 2 (Homo sapiens (Human)) | BDBM50079051 (6-[2-(4-Sulfamoyl-phenyl)-ethylcarbamoyl]-pyridine...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 7 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ degli Studi Curated by ChEMBL | Assay Description Inhibitory activity against human recombinant carbonic anhydrase II | J Med Chem 42: 2641-50 (1999) Article DOI: 10.1021/jm9900523 BindingDB Entry DOI: 10.7270/Q2J67HMK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carbonic anhydrase 2 (Homo sapiens (Human)) | BDBM50079049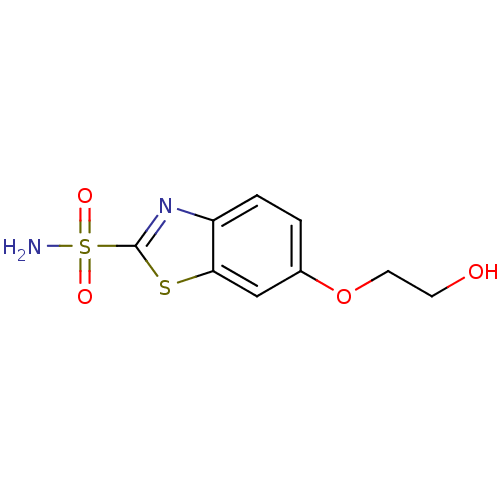 (6-(2-Hydroxy-ethoxy)-benzothiazole-2-sulfonic acid...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 7 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ degli Studi Curated by ChEMBL | Assay Description Inhibitory activity against human recombinant carbonic anhydrase II | J Med Chem 42: 2641-50 (1999) Article DOI: 10.1021/jm9900523 BindingDB Entry DOI: 10.7270/Q2J67HMK | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (docked) | ||||||||||||
| Carbonic anhydrase 2 (Homo sapiens (Human)) | BDBM50079058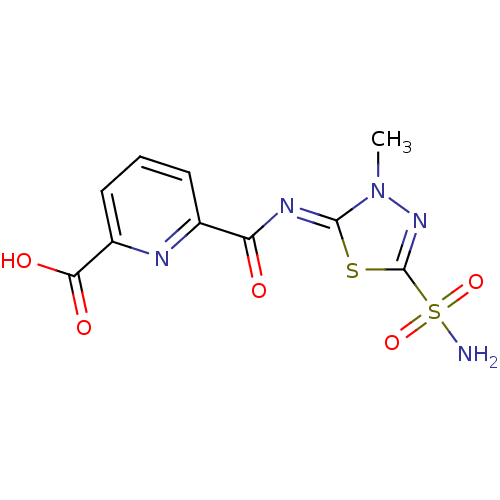 (6-[3-Methyl-5-sulfamoyl-3H-[1,3,4]thiadiazol-(2Z)-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | 7 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ degli Studi Curated by ChEMBL | Assay Description Inhibitory activity against human recombinant carbonic anhydrase II | J Med Chem 42: 2641-50 (1999) Article DOI: 10.1021/jm9900523 BindingDB Entry DOI: 10.7270/Q2J67HMK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carbonic anhydrase 2 (Homo sapiens (Human)) | BDBM50079033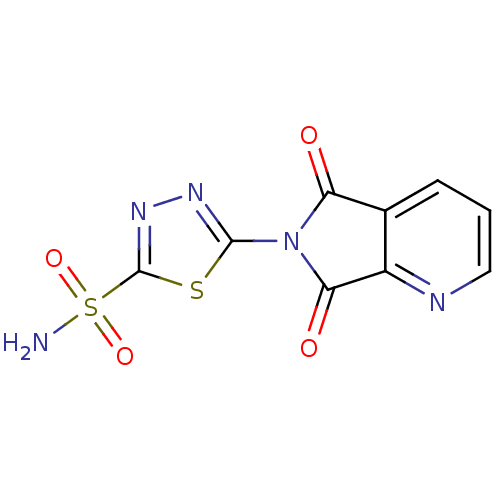 (5-(5,7-Dioxo-5,7-dihydro-pyrrolo[3,4-b]pyridin-6-y...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 8 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ degli Studi Curated by ChEMBL | Assay Description Inhibitory activity against human cloned Carbonic anhydrase II (hCA II,cytosolic form). | J Med Chem 42: 2641-50 (1999) Article DOI: 10.1021/jm9900523 BindingDB Entry DOI: 10.7270/Q2J67HMK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carbonic anhydrase 2 (Homo sapiens (Human)) | BDBM10874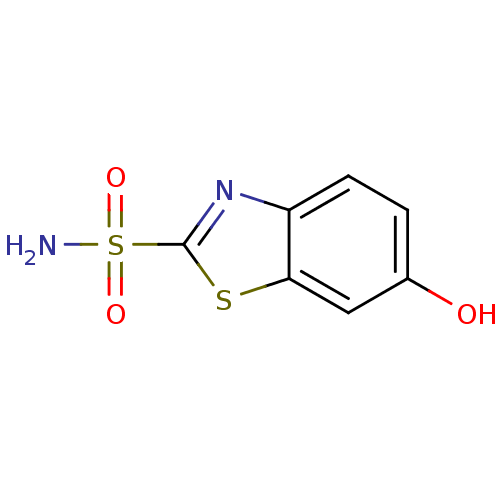 (6-hydroxy-1,3-benzothiazole-2-sulfonamide | CHEMBL...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MMDB PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | 8 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ degli Studi Curated by ChEMBL | Assay Description Inhibitory activity against human cloned Carbonic anhydrase II (hCA II,cytosolic form). | J Med Chem 42: 2641-50 (1999) Article DOI: 10.1021/jm9900523 BindingDB Entry DOI: 10.7270/Q2J67HMK | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Carbonic anhydrase 2 (Homo sapiens (Human)) | BDBM50079067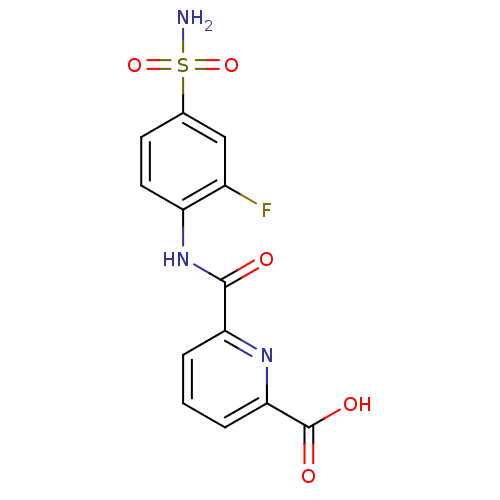 (6-(2-Fluoro-4-sulfamoyl-phenylcarbamoyl)-pyridine-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 8 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ degli Studi Curated by ChEMBL | Assay Description Inhibitory activity against human recombinant carbonic anhydrase II | J Med Chem 42: 2641-50 (1999) Article DOI: 10.1021/jm9900523 BindingDB Entry DOI: 10.7270/Q2J67HMK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carbonic anhydrase 2 (Homo sapiens (Human)) | BDBM50079052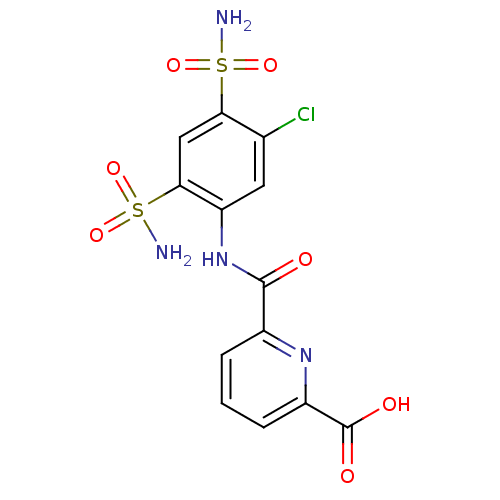 (6-(5-Chloro-2,4-disulfamoyl-phenylcarbamoyl)-pyrid...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 9 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ degli Studi Curated by ChEMBL | Assay Description Inhibitory activity against human recombinant carbonic anhydrase II | J Med Chem 42: 2641-50 (1999) Article DOI: 10.1021/jm9900523 BindingDB Entry DOI: 10.7270/Q2J67HMK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carbonic anhydrase 2 (Homo sapiens (Human)) | BDBM50079057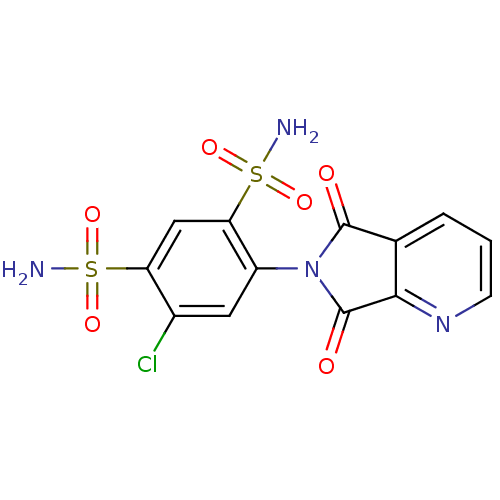 (4-Chloro-6-(5,7-dioxo-5,7-dihydro-pyrrolo[3,4-b]py...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 9 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ degli Studi Curated by ChEMBL | Assay Description Inhibitory activity against human cloned Carbonic anhydrase II (hCA II,cytosolic form). | J Med Chem 42: 2641-50 (1999) Article DOI: 10.1021/jm9900523 BindingDB Entry DOI: 10.7270/Q2J67HMK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carbonic anhydrase 2 (Homo sapiens (Human)) | BDBM50079027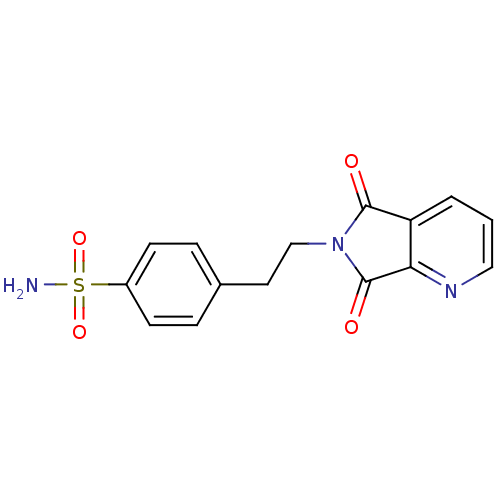 (4-[2-(5,7-Dioxo-5,7-dihydro-pyrrolo[3,4-b]pyridin-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 9 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ degli Studi Curated by ChEMBL | Assay Description Inhibitory activity against human recombinant carbonic anhydrase II | J Med Chem 42: 2641-50 (1999) Article DOI: 10.1021/jm9900523 BindingDB Entry DOI: 10.7270/Q2J67HMK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carbonic anhydrase 2 (Homo sapiens (Human)) | BDBM50079035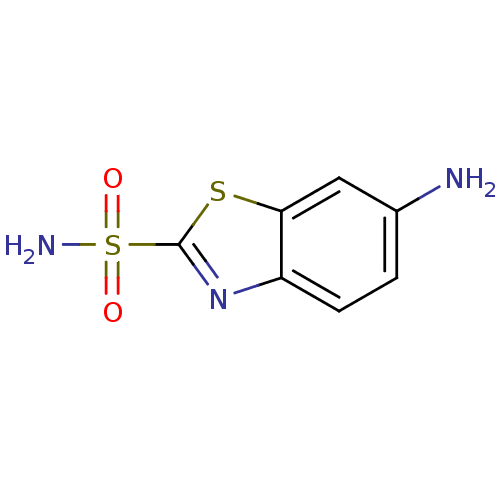 (6-Amino-benzothiazole-2-sulfonic acid amide | 6-am...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 9 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ degli Studi Curated by ChEMBL | Assay Description Inhibitory activity against human cloned Carbonic anhydrase II (hCA II,cytosolic form). | J Med Chem 42: 2641-50 (1999) Article DOI: 10.1021/jm9900523 BindingDB Entry DOI: 10.7270/Q2J67HMK | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (docked) | ||||||||||||
| Carbonic anhydrase 2 (Homo sapiens (Human)) | BDBM10884 ((2S,4S)-4-(ethylamino)-2-methyl-1,1-dioxo-2H,3H,4H...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | DrugBank PDB Article PubMed | 9 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ degli Studi Curated by ChEMBL | Assay Description Inhibitory activity against human recombinant carbonic anhydrase II | J Med Chem 42: 2641-50 (1999) Article DOI: 10.1021/jm9900523 BindingDB Entry DOI: 10.7270/Q2J67HMK | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Carbonic anhydrase 4 (Bos taurus (bovine)) | BDBM50079040 (6-[2-(5-Sulfamoyl-[1,3,4]thiadiazol-2-ylcarbamoyl)...) | Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 9 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ degli Studi Curated by ChEMBL | Assay Description Inhibitory activity against bovine lung microsomes carbonic anhydrase isozyme IV (bCA IV). | J Med Chem 42: 2641-50 (1999) Article DOI: 10.1021/jm9900523 BindingDB Entry DOI: 10.7270/Q2J67HMK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carbonic anhydrase 4 (Bos taurus (bovine)) | BDBM50079031 (6-(5,7-Dioxo-5,7-dihydro-pyrrolo[3,4-b]pyridin-6-y...) | Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | 9 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ degli Studi Curated by ChEMBL | Assay Description Inhibitory activity against bovine lung microsomes carbonic anhydrase isozyme IV (bCA IV). | J Med Chem 42: 2641-50 (1999) Article DOI: 10.1021/jm9900523 BindingDB Entry DOI: 10.7270/Q2J67HMK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carbonic anhydrase 4 (Bos taurus (bovine)) | BDBM50079062 (6-[2-(2-Sulfamoyl-benzothiazol-6-yloxy)-ethoxycarb...) | Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ degli Studi Curated by ChEMBL | Assay Description Inhibitory activity against bovine lung microsomes carbonic anhydrase isozyme IV (bCA IV). | J Med Chem 42: 2641-50 (1999) Article DOI: 10.1021/jm9900523 BindingDB Entry DOI: 10.7270/Q2J67HMK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carbonic anhydrase 4 (Bos taurus (bovine)) | BDBM50079042 (3-(5,7-Dioxo-5,7-dihydro-pyrrolo[3,4-b]pyridin-6-y...) | Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ degli Studi Curated by ChEMBL | Assay Description Inhibitory activity against bovine lung microsomes carbonic anhydrase isozyme IV (bCA IV). | J Med Chem 42: 2641-50 (1999) Article DOI: 10.1021/jm9900523 BindingDB Entry DOI: 10.7270/Q2J67HMK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carbonic anhydrase 2 (Homo sapiens (Human)) | BDBM50079063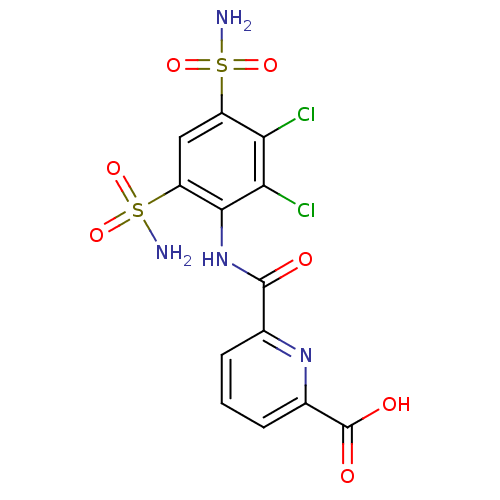 (6-(2,3-Dichloro-4,6-disulfamoyl-phenylcarbamoyl)-p...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ degli Studi Curated by ChEMBL | Assay Description Inhibitory activity against human recombinant carbonic anhydrase II | J Med Chem 42: 2641-50 (1999) Article DOI: 10.1021/jm9900523 BindingDB Entry DOI: 10.7270/Q2J67HMK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carbonic anhydrase 2 (Homo sapiens (Human)) | BDBM50079030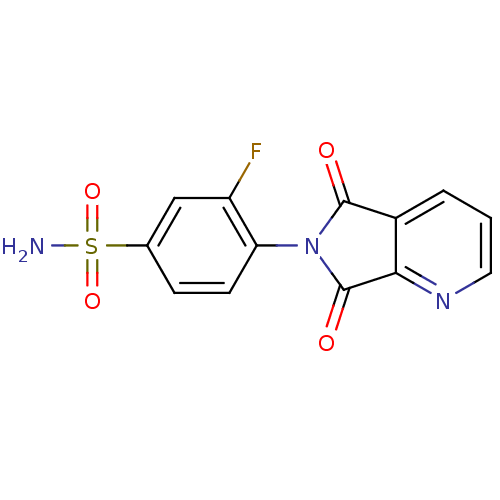 (4-(5,7-Dioxo-5,7-dihydro-pyrrolo[3,4-b]pyridin-6-y...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ degli Studi Curated by ChEMBL | Assay Description Inhibitory activity against human recombinant carbonic anhydrase II | J Med Chem 42: 2641-50 (1999) Article DOI: 10.1021/jm9900523 BindingDB Entry DOI: 10.7270/Q2J67HMK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carbonic anhydrase 4 (Bos taurus (bovine)) | BDBM50079034 (6-(2-Sulfamoyl-benzothiazol-6-ylcarbamoyl)-pyridin...) | Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | 11 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ degli Studi Curated by ChEMBL | Assay Description Inhibitory activity against bovine lung microsomes carbonic anhydrase isozyme IV (bCA IV). | J Med Chem 42: 2641-50 (1999) Article DOI: 10.1021/jm9900523 BindingDB Entry DOI: 10.7270/Q2J67HMK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carbonic anhydrase 1 (Homo sapiens (Human)) | BDBM50079031 (6-(5,7-Dioxo-5,7-dihydro-pyrrolo[3,4-b]pyridin-6-y...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | 12 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ degli Studi Curated by ChEMBL | Assay Description Inhibition of human cloned Carbonic anhydrase I (hCA I,cytosolic form) | J Med Chem 42: 2641-50 (1999) Article DOI: 10.1021/jm9900523 BindingDB Entry DOI: 10.7270/Q2J67HMK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carbonic anhydrase 2 (Homo sapiens (Human)) | BDBM50079041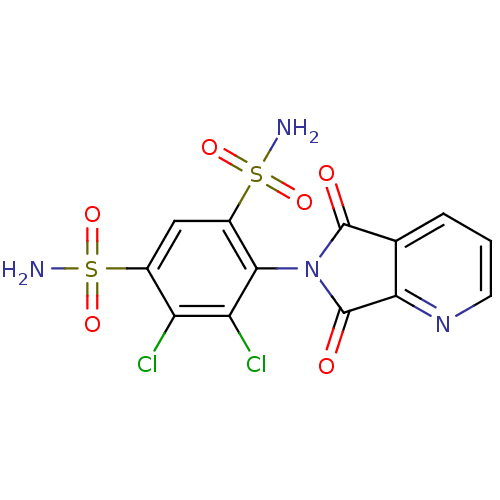 (4,5-Dichloro-6-(5,7-dioxo-5,7-dihydro-pyrrolo[3,4-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 12 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ degli Studi Curated by ChEMBL | Assay Description Inhibitory activity against human cloned Carbonic anhydrase II (hCA II,cytosolic form). | J Med Chem 42: 2641-50 (1999) Article DOI: 10.1021/jm9900523 BindingDB Entry DOI: 10.7270/Q2J67HMK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carbonic anhydrase 4 (Bos taurus (bovine)) | BDBM50079059 (6-(2-Sulfamoyl-benzothiazol-6-yloxycarbamoyl)-pyri...) | Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 13 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ degli Studi Curated by ChEMBL | Assay Description Inhibitory activity against bovine lung microsomes carbonic anhydrase isozyme IV (bCA IV). | J Med Chem 42: 2641-50 (1999) Article DOI: 10.1021/jm9900523 BindingDB Entry DOI: 10.7270/Q2J67HMK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carbonic anhydrase 4 (Bos taurus (bovine)) | BDBM50079049 (6-(2-Hydroxy-ethoxy)-benzothiazole-2-sulfonic acid...) | Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 15 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ degli Studi Curated by ChEMBL | Assay Description Inhibitory activity against bovine lung microsomes carbonic anhydrase isozyme IV (bCA IV). | J Med Chem 42: 2641-50 (1999) Article DOI: 10.1021/jm9900523 BindingDB Entry DOI: 10.7270/Q2J67HMK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carbonic anhydrase 2 (Homo sapiens (Human)) | BDBM50079066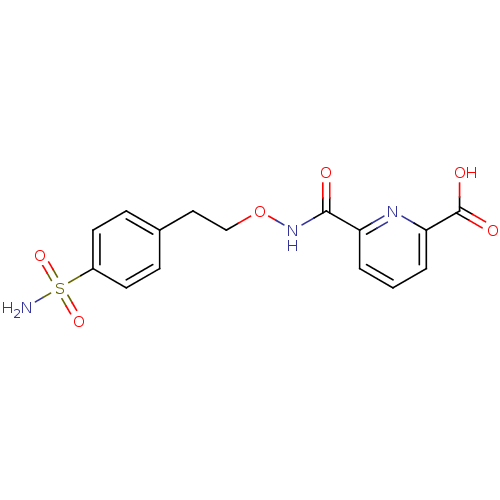 (6-[2-(4-Sulfamoyl-phenyl)-ethoxycarbamoyl]-pyridin...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 15 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ degli Studi Curated by ChEMBL | Assay Description Inhibitory activity against human recombinant carbonic anhydrase II | J Med Chem 42: 2641-50 (1999) Article DOI: 10.1021/jm9900523 BindingDB Entry DOI: 10.7270/Q2J67HMK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carbonic anhydrase 1 (Homo sapiens (Human)) | BDBM50079062 (6-[2-(2-Sulfamoyl-benzothiazol-6-yloxy)-ethoxycarb...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 15 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ degli Studi Curated by ChEMBL | Assay Description Inhibitory activity against human cloned carbonic anhydrase isozyme I (hCA I, cytosolic form). | J Med Chem 42: 2641-50 (1999) Article DOI: 10.1021/jm9900523 BindingDB Entry DOI: 10.7270/Q2J67HMK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carbonic anhydrase 1 (Homo sapiens (Human)) | BDBM50079059 (6-(2-Sulfamoyl-benzothiazol-6-yloxycarbamoyl)-pyri...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 16 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ degli Studi Curated by ChEMBL | Assay Description Inhibitory activity against human cloned carbonic anhydrase isozyme I (hCA I, cytosolic form). | J Med Chem 42: 2641-50 (1999) Article DOI: 10.1021/jm9900523 BindingDB Entry DOI: 10.7270/Q2J67HMK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carbonic anhydrase 4 (Bos taurus (bovine)) | BDBM10874 (6-hydroxy-1,3-benzothiazole-2-sulfonamide | CHEMBL...) | Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL DrugBank MMDB PC cid PC sid PDB UniChem Patents Similars | Article PubMed | 17 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ degli Studi Curated by ChEMBL | Assay Description Inhibitory activity against bovine lung microsomes carbonic anhydrase isozyme IV (bCA IV). | J Med Chem 42: 2641-50 (1999) Article DOI: 10.1021/jm9900523 BindingDB Entry DOI: 10.7270/Q2J67HMK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carbonic anhydrase 4 (Bos taurus (bovine)) | BDBM50079048 (6-[Ethyl-(6-methyl-7,7-dioxo-2-sulfamoyl-4,5,6,7-t...) | Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 17 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ degli Studi Curated by ChEMBL | Assay Description Inhibitory activity against bovine lung microsomes carbonic anhydrase isozyme IV (bCA IV). | J Med Chem 42: 2641-50 (1999) Article DOI: 10.1021/jm9900523 BindingDB Entry DOI: 10.7270/Q2J67HMK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carbonic anhydrase 4 (Bos taurus (bovine)) | BDBM50079056 (2-[Ethyl-(6-methyl-7,7-dioxo-2-sulfamoyl-4,5,6,7-t...) | Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 18 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ degli Studi Curated by ChEMBL | Assay Description Inhibitory activity against bovine lung microsomes carbonic anhydrase isozyme IV (bCA IV). | J Med Chem 42: 2641-50 (1999) Article DOI: 10.1021/jm9900523 BindingDB Entry DOI: 10.7270/Q2J67HMK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carbonic anhydrase 4 (Bos taurus (bovine)) | BDBM50079033 (5-(5,7-Dioxo-5,7-dihydro-pyrrolo[3,4-b]pyridin-6-y...) | Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 18 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ degli Studi Curated by ChEMBL | Assay Description Inhibitory activity against bovine lung microsomes carbonic anhydrase isozyme IV (bCA IV). | J Med Chem 42: 2641-50 (1999) Article DOI: 10.1021/jm9900523 BindingDB Entry DOI: 10.7270/Q2J67HMK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carbonic anhydrase 1 (Homo sapiens (Human)) | BDBM50079034 (6-(2-Sulfamoyl-benzothiazol-6-ylcarbamoyl)-pyridin...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | 18 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ degli Studi Curated by ChEMBL | Assay Description Inhibitory activity against human cloned carbonic anhydrase isozyme I (hCA I, cytosolic form). | J Med Chem 42: 2641-50 (1999) Article DOI: 10.1021/jm9900523 BindingDB Entry DOI: 10.7270/Q2J67HMK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carbonic anhydrase 1 (Homo sapiens (Human)) | BDBM50079042 (3-(5,7-Dioxo-5,7-dihydro-pyrrolo[3,4-b]pyridin-6-y...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 18 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ degli Studi Curated by ChEMBL | Assay Description Inhibition of human cloned Carbonic anhydrase I (hCA I,cytosolic form) | J Med Chem 42: 2641-50 (1999) Article DOI: 10.1021/jm9900523 BindingDB Entry DOI: 10.7270/Q2J67HMK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carbonic anhydrase 2 (Homo sapiens (Human)) | BDBM10869 (5-imino-4-methyl-4,5-dihydro-1,3,4-thiadiazole-2-s...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents | Article PubMed | 19 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ degli Studi Curated by ChEMBL | Assay Description Inhibitory activity against human recombinant carbonic anhydrase II | J Med Chem 42: 2641-50 (1999) Article DOI: 10.1021/jm9900523 BindingDB Entry DOI: 10.7270/Q2J67HMK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carbonic anhydrase 4 (Bos taurus (bovine)) | BDBM50079035 (6-Amino-benzothiazole-2-sulfonic acid amide | 6-am...) | Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 19 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ degli Studi Curated by ChEMBL | Assay Description Inhibitory activity against bovine lung microsomes carbonic anhydrase isozyme IV (bCA IV). | J Med Chem 42: 2641-50 (1999) Article DOI: 10.1021/jm9900523 BindingDB Entry DOI: 10.7270/Q2J67HMK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carbonic anhydrase 4 (Bos taurus (bovine)) | BDBM50079058 (6-[3-Methyl-5-sulfamoyl-3H-[1,3,4]thiadiazol-(2Z)-...) | Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | 19 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ degli Studi Curated by ChEMBL | Assay Description Inhibitory activity against bovine lung microsomes carbonic anhydrase isozyme IV (bCA IV). | J Med Chem 42: 2641-50 (1999) Article DOI: 10.1021/jm9900523 BindingDB Entry DOI: 10.7270/Q2J67HMK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carbonic anhydrase 1 (Homo sapiens (Human)) | BDBM50079040 (6-[2-(5-Sulfamoyl-[1,3,4]thiadiazol-2-ylcarbamoyl)...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 19 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ degli Studi Curated by ChEMBL | Assay Description Inhibitory activity against human cloned carbonic anhydrase isozyme I (hCA I, cytosolic form). | J Med Chem 42: 2641-50 (1999) Article DOI: 10.1021/jm9900523 BindingDB Entry DOI: 10.7270/Q2J67HMK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carbonic anhydrase 2 (Homo sapiens (Human)) | BDBM50079044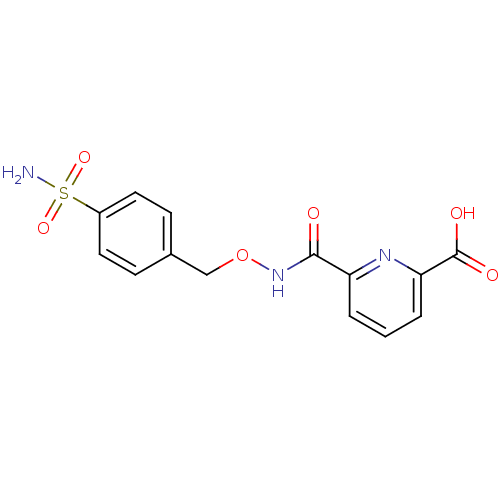 (6-(4-Sulfamoyl-benzyloxycarbamoyl)-pyridine-2-carb...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ degli Studi Curated by ChEMBL | Assay Description Inhibitory activity against human recombinant carbonic anhydrase II | J Med Chem 42: 2641-50 (1999) Article DOI: 10.1021/jm9900523 BindingDB Entry DOI: 10.7270/Q2J67HMK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carbonic anhydrase 4 (Bos taurus (bovine)) | BDBM50079037 (6-(5-Sulfamoyl-[1,3,4]thiadiazol-2-ylcarbamoyl)-py...) | Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 21 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ degli Studi Curated by ChEMBL | Assay Description Inhibitory activity against bovine lung microsomes carbonic anhydrase isozyme IV (bCA IV). | J Med Chem 42: 2641-50 (1999) Article DOI: 10.1021/jm9900523 BindingDB Entry DOI: 10.7270/Q2J67HMK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carbonic anhydrase 2 (Homo sapiens (Human)) | BDBM50079038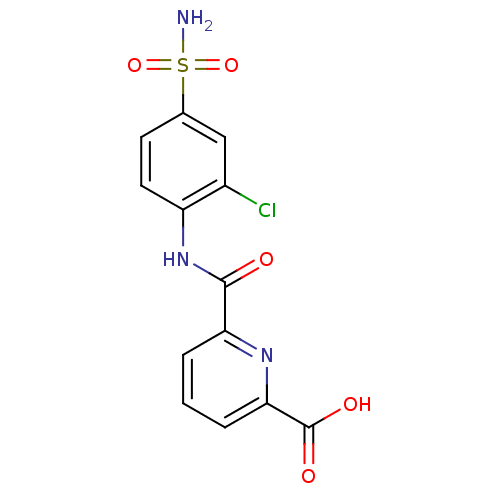 (6-(2-Chloro-4-sulfamoyl-phenylcarbamoyl)-pyridine-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 25 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ degli Studi Curated by ChEMBL | Assay Description Inhibitory activity against human cloned Carbonic anhydrase II (hCA II,cytosolic form). | J Med Chem 42: 2641-50 (1999) Article DOI: 10.1021/jm9900523 BindingDB Entry DOI: 10.7270/Q2J67HMK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carbonic anhydrase 2 (Homo sapiens (Human)) | BDBM50079043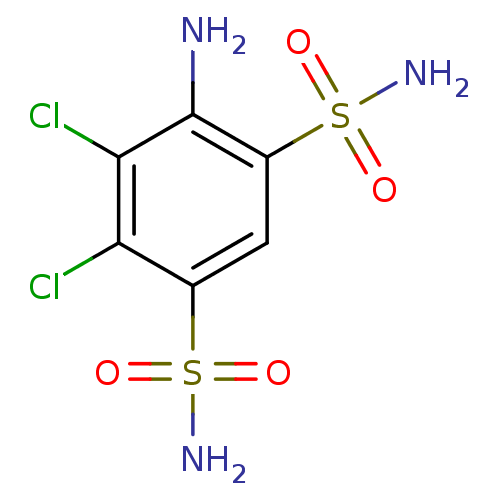 (4-Amino-5,6-dichloro-benzene-1,3-disulfonic acid d...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Similars | Article PubMed | 28 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ degli Studi Curated by ChEMBL | Assay Description Inhibitory activity against human recombinant carbonic anhydrase II | J Med Chem 42: 2641-50 (1999) Article DOI: 10.1021/jm9900523 BindingDB Entry DOI: 10.7270/Q2J67HMK | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (docked) | ||||||||||||
| Displayed 1 to 50 (of 176 total ) | Next | Last >> |