

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
BDBM50079063 6-(2,3-Dichloro-4,6-disulfamoyl-phenylcarbamoyl)-pyridine-2-carboxylic acid::BDBM50222604::CHEMBL89634
SMILES: NS(=O)(=O)c1cc(c(NC(=O)c2cccc(n2)C(O)=O)c(Cl)c1Cl)S(N)(=O)=O
InChI Key: InChIKey=IVKDUEUEOMQWRK-UHFFFAOYSA-N
Data: 6 KI
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kcal/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Carbonic anhydrase 2 (Homo sapiens (Human)) | BDBM50079063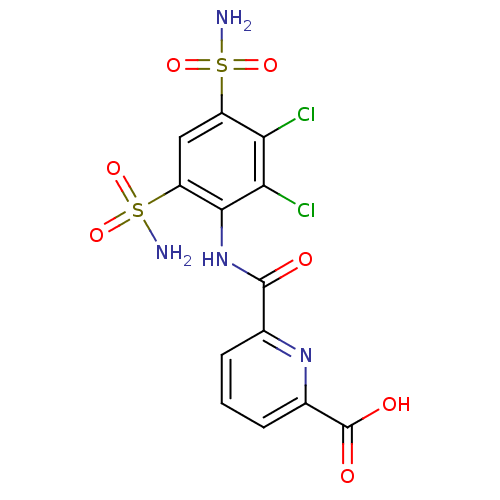 (6-(2,3-Dichloro-4,6-disulfamoyl-phenylcarbamoyl)-p...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt UniProtKB/TrEMBL B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ degli Studi Curated by ChEMBL | Assay Description Inhibitory activity against human recombinant carbonic anhydrase II | J Med Chem 42: 2641-50 (1999) Article DOI: 10.1021/jm9900523 BindingDB Entry DOI: 10.7270/Q2J67HMK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carbonic anhydrase 2 (Homo sapiens (Human)) | BDBM50079063 (6-(2,3-Dichloro-4,6-disulfamoyl-phenylcarbamoyl)-p...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt UniProtKB/TrEMBL B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
A.P.S. University Curated by ChEMBL | Assay Description Inhibition of human carbonic anhydrase II (CAII) | Bioorg Med Chem Lett 13: 447-53 (2003) BindingDB Entry DOI: 10.7270/Q2668GC9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carbonic anhydrase (Bos taurus (bovine)) | BDBM50079063 (6-(2,3-Dichloro-4,6-disulfamoyl-phenylcarbamoyl)-p...) | Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 55 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
A.P.S. University Curated by ChEMBL | Assay Description Inhibition of bovine carbonic anhydrase IV (CAIV) | Bioorg Med Chem Lett 13: 447-53 (2003) BindingDB Entry DOI: 10.7270/Q2668GC9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carbonic Anhydrase IV (Bos taurus (bovine)) | BDBM50079063 (6-(2,3-Dichloro-4,6-disulfamoyl-phenylcarbamoyl)-p...) | Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 55 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ degli Studi Curated by ChEMBL | Assay Description Inhibitory activity against bovine lung microsomes carbonic anhydrase isozyme IV (bCA IV). | J Med Chem 42: 2641-50 (1999) Article DOI: 10.1021/jm9900523 BindingDB Entry DOI: 10.7270/Q2J67HMK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carbonic anhydrase 1 (Homo sapiens (Human)) | BDBM50079063 (6-(2,3-Dichloro-4,6-disulfamoyl-phenylcarbamoyl)-p...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt UniProtKB/TrEMBL B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 450 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
A.P.S. University Curated by ChEMBL | Assay Description Inhibition of human carbonic anhydrase I (CAI) | Bioorg Med Chem Lett 13: 447-53 (2003) BindingDB Entry DOI: 10.7270/Q2668GC9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carbonic anhydrase 1 (Homo sapiens (Human)) | BDBM50079063 (6-(2,3-Dichloro-4,6-disulfamoyl-phenylcarbamoyl)-p...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt UniProtKB/TrEMBL B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 450 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ degli Studi Curated by ChEMBL | Assay Description Inhibitory activity against human cloned carbonic anhydrase isozyme I (hCA I, cytosolic form). | J Med Chem 42: 2641-50 (1999) Article DOI: 10.1021/jm9900523 BindingDB Entry DOI: 10.7270/Q2J67HMK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||