

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Neprilysin (Homo sapiens (Human)) | BDBM50105262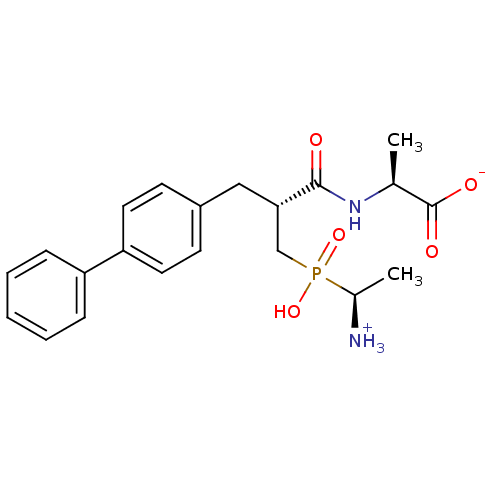 (2-{3-[(1-Amino-ethyl)-hydroxy-phosphinoyl]-2-biphe...) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 1.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
INSERM Curated by ChEMBL | Assay Description Compound was evaluated for inhibition of enkephalin degrading enzyme, neutral endopeptidase (NEP) | J Med Chem 44: 3523-30 (2001) BindingDB Entry DOI: 10.7270/Q2QN67HK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neprilysin (Homo sapiens (Human)) | BDBM50105264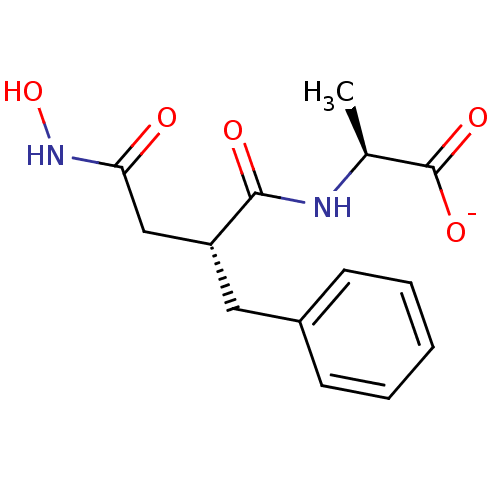 (2-(2-Benzyl-3-hydroxycarbamoyl-propionylamino)-pro...) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid PDB UniChem Similars | PubMed | 1.70 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
INSERM Curated by ChEMBL | Assay Description Compound was evaluated for inhibition of enkephalin degrading enzyme, neutral endopeptidase (NEP) | J Med Chem 44: 3523-30 (2001) BindingDB Entry DOI: 10.7270/Q2QN67HK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neprilysin (Homo sapiens (Human)) | BDBM50105261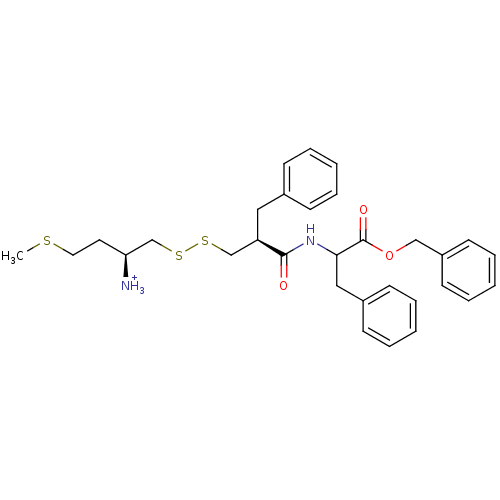 (1-[2-(1-Benzyloxycarbonyl-2-phenyl-ethylcarbamoyl)...) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | KEGG PC cid PC sid UniChem | PubMed | 2 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
INSERM Curated by ChEMBL | Assay Description Compound was evaluated for inhibition of enkephalin degrading enzyme, neutral endopeptidase (NEP) | J Med Chem 44: 3523-30 (2001) BindingDB Entry DOI: 10.7270/Q2QN67HK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neprilysin (Homo sapiens (Human)) | BDBM50105263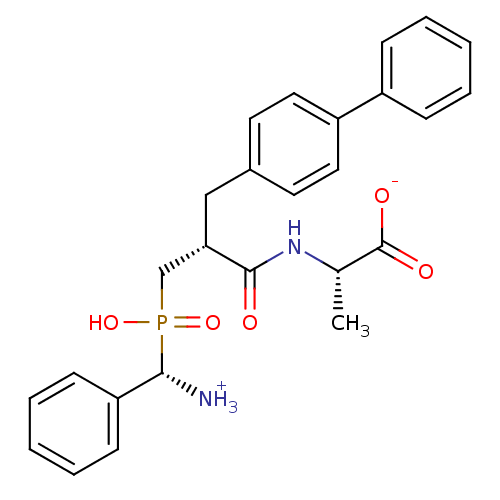 (2-{3-[(Amino-phenyl-methyl)-hydroxy-phosphinoyl]-2...) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 2 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
INSERM Curated by ChEMBL | Assay Description Compound was evaluated for inhibition of enkephalin degrading enzyme, neutral endopeptidase (NEP) | J Med Chem 44: 3523-30 (2001) BindingDB Entry DOI: 10.7270/Q2QN67HK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aminopeptidase N (Homo sapiens (Human)) | BDBM50105262 (2-{3-[(1-Amino-ethyl)-hydroxy-phosphinoyl]-2-biphe...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 2.90 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
INSERM Curated by ChEMBL | Assay Description Compound was evaluated for inhibition of enkephalin degrading enzyme, aminopeptidase N(APN) | J Med Chem 44: 3523-30 (2001) BindingDB Entry DOI: 10.7270/Q2QN67HK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aminopeptidase N (Homo sapiens (Human)) | BDBM50105263 (2-{3-[(Amino-phenyl-methyl)-hydroxy-phosphinoyl]-2...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 4.80 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
INSERM Curated by ChEMBL | Assay Description Compound was evaluated for inhibition of enkephalin degrading enzyme, aminopeptidase N(APN) | J Med Chem 44: 3523-30 (2001) BindingDB Entry DOI: 10.7270/Q2QN67HK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aminopeptidase N (Homo sapiens (Human)) | BDBM50105261 (1-[2-(1-Benzyloxycarbonyl-2-phenyl-ethylcarbamoyl)...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | KEGG PC cid PC sid UniChem | PubMed | 11 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
INSERM Curated by ChEMBL | Assay Description Compound was evaluated for inhibition of enkephalin degrading enzyme, aminopeptidase N(APN) | J Med Chem 44: 3523-30 (2001) BindingDB Entry DOI: 10.7270/Q2QN67HK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aminopeptidase N (Homo sapiens (Human)) | BDBM50105264 (2-(2-Benzyl-3-hydroxycarbamoyl-propionylamino)-pro...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid PDB UniChem Similars | PubMed | 7.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
INSERM Curated by ChEMBL | Assay Description Compound was evaluated for inhibition of enkephalin degrading enzyme, aminopeptidase N(APN) | J Med Chem 44: 3523-30 (2001) BindingDB Entry DOI: 10.7270/Q2QN67HK | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (docked) | ||||||||||||