 Found 80 hits Enz. Inhib. hit(s) with all data for entry = 50038376
Found 80 hits Enz. Inhib. hit(s) with all data for entry = 50038376 Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Bile acid receptor
(Homo sapiens (Human)) | BDBM50423620
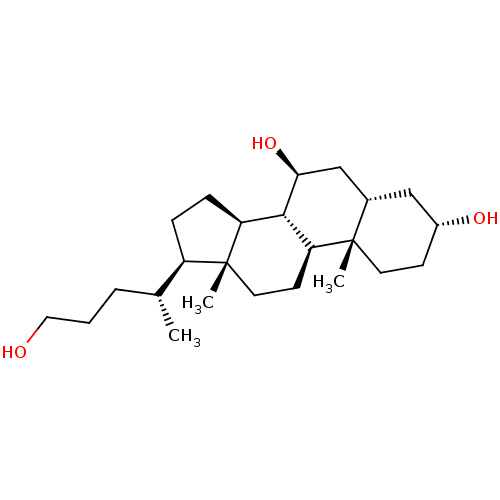
(CHEMBL1253814)Show SMILES C[C@H](CCCO)[C@H]1CC[C@H]2[C@@H]3[C@@H](O)C[C@@H]4C[C@H](O)CC[C@]4(C)[C@H]3CC[C@]12C |r| Show InChI InChI=1S/C24H42O3/c1-15(5-4-12-25)18-6-7-19-22-20(9-11-24(18,19)3)23(2)10-8-17(26)13-16(23)14-21(22)27/h15-22,25-27H,4-14H2,1-3H3/t15-,16+,17-,18-,19+,20+,21+,22+,23+,24-/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | >3.16E+4 | n/a | n/a | n/a | n/a |
INSERM
Curated by ChEMBL
| Assay Description
Agonist activity at human FXR expressed in COS1 cells by luciferase assay |
J Med Chem 51: 1831-41 (2008)
Article DOI: 10.1021/jm7015864
BindingDB Entry DOI: 10.7270/Q2222VNJ |
More data for this
Ligand-Target Pair | |
G-protein coupled bile acid receptor 1
(Homo sapiens (Human)) | BDBM50423620

(CHEMBL1253814)Show SMILES C[C@H](CCCO)[C@H]1CC[C@H]2[C@@H]3[C@@H](O)C[C@@H]4C[C@H](O)CC[C@]4(C)[C@H]3CC[C@]12C |r| Show InChI InChI=1S/C24H42O3/c1-15(5-4-12-25)18-6-7-19-22-20(9-11-24(18,19)3)23(2)10-8-17(26)13-16(23)14-21(22)27/h15-22,25-27H,4-14H2,1-3H3/t15-,16+,17-,18-,19+,20+,21+,22+,23+,24-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 2.18E+3 | n/a | n/a | n/a | n/a |
INSERM
Curated by ChEMBL
| Assay Description
Agonist activity at human TGR5 expressed in CHO cells by luciferase assay |
J Med Chem 51: 1831-41 (2008)
Article DOI: 10.1021/jm7015864
BindingDB Entry DOI: 10.7270/Q2222VNJ |
More data for this
Ligand-Target Pair | |
G-protein coupled bile acid receptor 1
(Homo sapiens (Human)) | BDBM50334958
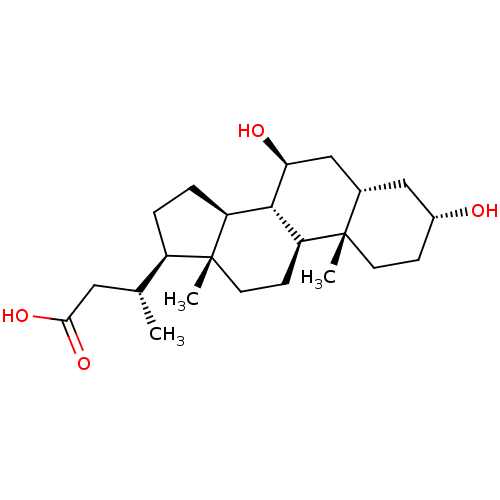
((R)-3-((3R,5S,7S,8R,9S,10S,13R,14S,17R)-3,7-dihydr...)Show SMILES C[C@H](CC(O)=O)[C@H]1CC[C@H]2[C@@H]3[C@@H](O)C[C@@H]4C[C@H](O)CC[C@]4(C)[C@H]3CC[C@]12C |r| Show InChI InChI=1S/C23H38O4/c1-13(10-20(26)27)16-4-5-17-21-18(7-9-23(16,17)3)22(2)8-6-15(24)11-14(22)12-19(21)25/h13-19,21,24-25H,4-12H2,1-3H3,(H,26,27)/t13-,14+,15-,16-,17+,18+,19+,21+,22+,23-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 4.72E+4 | n/a | n/a | n/a | n/a |
INSERM
Curated by ChEMBL
| Assay Description
Agonist activity at human TGR5 expressed in CHO cells by luciferase assay |
J Med Chem 51: 1831-41 (2008)
Article DOI: 10.1021/jm7015864
BindingDB Entry DOI: 10.7270/Q2222VNJ |
More data for this
Ligand-Target Pair | |
G-protein coupled bile acid receptor 1
(Homo sapiens (Human)) | BDBM50423621

(CHEMBL1609669 | lago-DCA)Show SMILES CC(CCC(O)=O)C1CCC2C3CC[C@H]4CC(O)CC[C@@]4(C)C3CC(O)[C@]12C Show InChI InChI=1S/C24H40O4/c1-14(4-9-22(27)28)18-7-8-19-17-6-5-15-12-16(25)10-11-23(15,2)20(17)13-21(26)24(18,19)3/h14-21,25-26H,4-13H2,1-3H3,(H,27,28)/t14?,15-,16?,17?,18?,19?,20?,21?,23+,24+/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 4.39E+3 | n/a | n/a | n/a | n/a |
INSERM
Curated by ChEMBL
| Assay Description
Agonist activity at human TGR5 expressed in CHO cells by luciferase assay |
J Med Chem 51: 1831-41 (2008)
Article DOI: 10.1021/jm7015864
BindingDB Entry DOI: 10.7270/Q2222VNJ |
More data for this
Ligand-Target Pair | |
Bile acid receptor
(Homo sapiens (Human)) | BDBM50375549
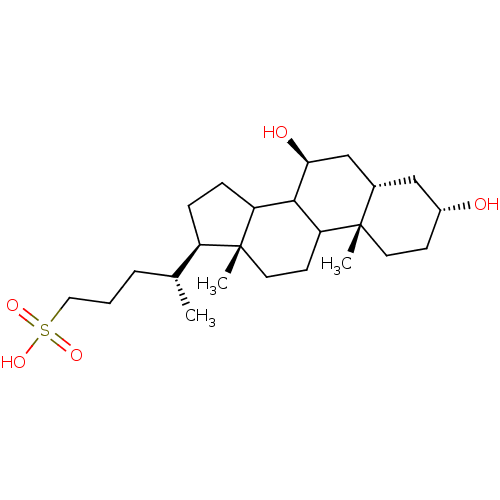
(CHEMBL1628228)Show SMILES C[C@H](CCCS(O)(=O)=O)[C@H]1CCC2C3[C@@H](O)C[C@@H]4C[C@H](O)CC[C@]4(C)C3CC[C@]12C |r| Show InChI InChI=1S/C24H42O5S/c1-15(5-4-12-30(27,28)29)18-6-7-19-22-20(9-11-24(18,19)3)23(2)10-8-17(25)13-16(23)14-21(22)26/h15-22,25-26H,4-14H2,1-3H3,(H,27,28,29)/t15-,16+,17-,18-,19?,20?,21+,22?,23+,24-/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
KEGG
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | >1.00E+5 | n/a | n/a | n/a | n/a |
INSERM
Curated by ChEMBL
| Assay Description
Agonist activity at human FXR expressed in COS1 cells by luciferase assay |
J Med Chem 51: 1831-41 (2008)
Article DOI: 10.1021/jm7015864
BindingDB Entry DOI: 10.7270/Q2222VNJ |
More data for this
Ligand-Target Pair | |
Bile acid receptor
(Homo sapiens (Human)) | BDBM50375551
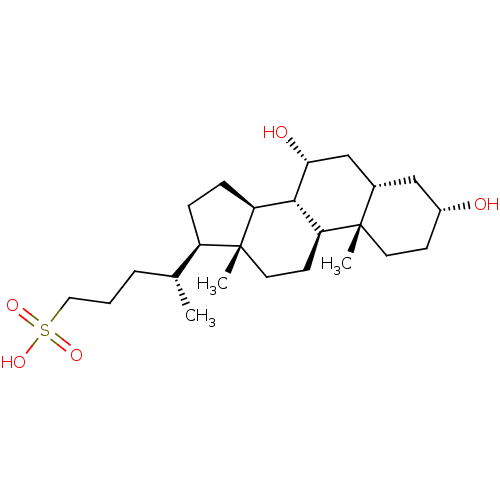
(CHEMBL406923)Show SMILES C[C@H](CCCS(O)(=O)=O)[C@H]1CC[C@H]2[C@@H]3[C@H](O)C[C@@H]4C[C@H](O)CC[C@]4(C)[C@H]3CC[C@]12C Show InChI InChI=1S/C24H42O5S/c1-15(5-4-12-30(27,28)29)18-6-7-19-22-20(9-11-24(18,19)3)23(2)10-8-17(25)13-16(23)14-21(22)26/h15-22,25-26H,4-14H2,1-3H3,(H,27,28,29)/t15-,16+,17-,18-,19+,20+,21-,22+,23+,24-/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
KEGG
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | >1.00E+5 | n/a | n/a | n/a | n/a |
INSERM
Curated by ChEMBL
| Assay Description
Agonist activity at human FXR expressed in COS1 cells by luciferase assay |
J Med Chem 51: 1831-41 (2008)
Article DOI: 10.1021/jm7015864
BindingDB Entry DOI: 10.7270/Q2222VNJ |
More data for this
Ligand-Target Pair | |
Bile acid receptor
(Homo sapiens (Human)) | BDBM50375552
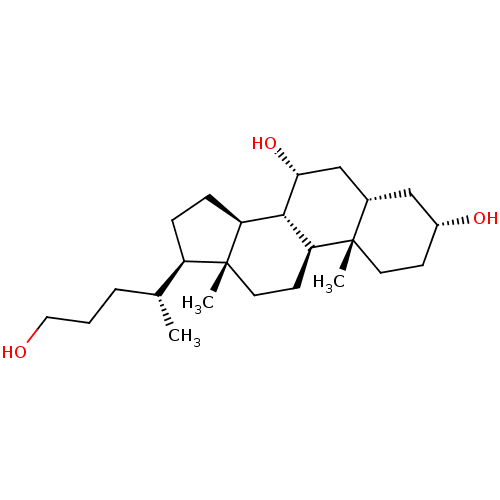
(CHEMBL270287)Show SMILES C[C@H](CCCO)[C@H]1CC[C@H]2[C@@H]3[C@H](O)C[C@@H]4C[C@H](O)CC[C@]4(C)[C@H]3CC[C@]12C Show InChI InChI=1S/C24H42O3/c1-15(5-4-12-25)18-6-7-19-22-20(9-11-24(18,19)3)23(2)10-8-17(26)13-16(23)14-21(22)27/h15-22,25-27H,4-14H2,1-3H3/t15-,16+,17-,18-,19+,20+,21-,22+,23+,24-/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 6.00E+3 | n/a | n/a | n/a | n/a |
INSERM
Curated by ChEMBL
| Assay Description
Agonist activity at human FXR expressed in COS1 cells by luciferase assay |
J Med Chem 51: 1831-41 (2008)
Article DOI: 10.1021/jm7015864
BindingDB Entry DOI: 10.7270/Q2222VNJ |
More data for this
Ligand-Target Pair | |
Bile acid receptor
(Homo sapiens (Human)) | BDBM50375553
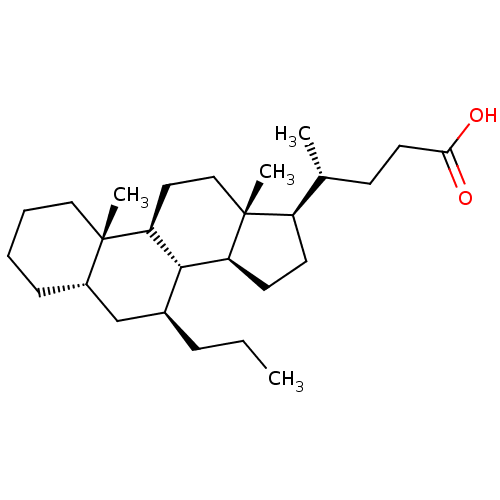
(CHEMBL405879)Show SMILES CCC[C@H]1C[C@@H]2CCCC[C@]2(C)[C@H]2CC[C@]3(C)[C@H](CC[C@H]3[C@H]12)[C@H](C)CCC(O)=O Show InChI InChI=1S/C27H46O2/c1-5-8-19-17-20-9-6-7-15-26(20,3)23-14-16-27(4)21(11-12-22(27)25(19)23)18(2)10-13-24(28)29/h18-23,25H,5-17H2,1-4H3,(H,28,29)/t18-,19+,20+,21-,22+,23+,25+,26+,27-/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | >3.16E+4 | n/a | n/a | n/a | n/a |
INSERM
Curated by ChEMBL
| Assay Description
Agonist activity at human FXR expressed in COS1 cells by luciferase assay |
J Med Chem 51: 1831-41 (2008)
Article DOI: 10.1021/jm7015864
BindingDB Entry DOI: 10.7270/Q2222VNJ |
More data for this
Ligand-Target Pair | |
Bile acid receptor
(Homo sapiens (Human)) | BDBM50375554
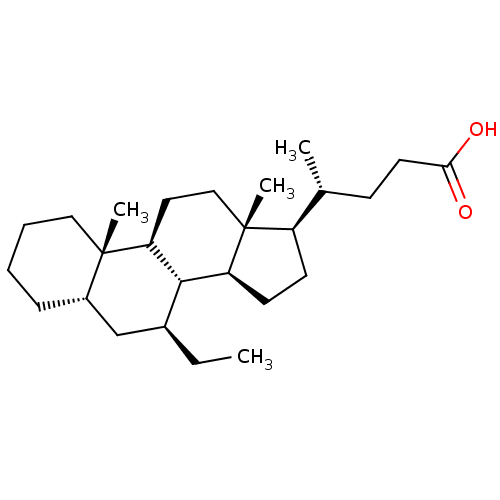
(CHEMBL270494)Show SMILES CC[C@H]1C[C@@H]2CCCC[C@]2(C)[C@H]2CC[C@]3(C)[C@H](CC[C@H]3[C@H]12)[C@H](C)CCC(O)=O Show InChI InChI=1S/C26H44O2/c1-5-18-16-19-8-6-7-14-25(19,3)22-13-15-26(4)20(10-11-21(26)24(18)22)17(2)9-12-23(27)28/h17-22,24H,5-16H2,1-4H3,(H,27,28)/t17-,18+,19+,20-,21+,22+,24+,25+,26-/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | >5.00E+4 | n/a | n/a | n/a | n/a |
INSERM
Curated by ChEMBL
| Assay Description
Agonist activity at human FXR expressed in COS1 cells by luciferase assay |
J Med Chem 51: 1831-41 (2008)
Article DOI: 10.1021/jm7015864
BindingDB Entry DOI: 10.7270/Q2222VNJ |
More data for this
Ligand-Target Pair | |
Bile acid receptor
(Homo sapiens (Human)) | BDBM50375555
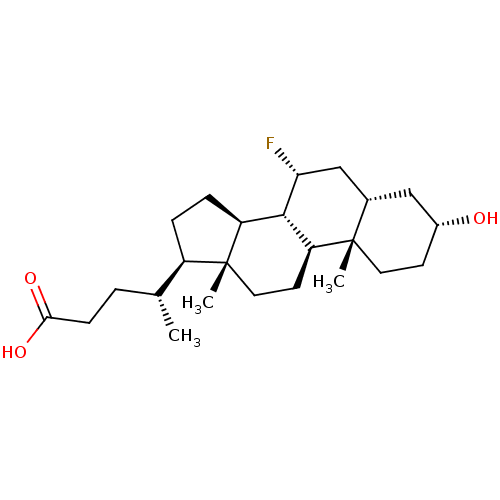
(3 ALPHA-HYDROXY-7 ALPHA-FLUORO-5 BETA-CHOLAN-24-OI...)Show SMILES C[C@H](CCC(O)=O)[C@H]1CC[C@H]2[C@@H]3[C@H](F)C[C@@H]4C[C@H](O)CC[C@]4(C)[C@H]3CC[C@]12C Show InChI InChI=1S/C24H39FO3/c1-14(4-7-21(27)28)17-5-6-18-22-19(9-11-24(17,18)3)23(2)10-8-16(26)12-15(23)13-20(22)25/h14-20,22,26H,4-13H2,1-3H3,(H,27,28)/t14-,15+,16-,17-,18+,19+,20-,22+,23+,24-/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | >1.00E+5 | n/a | n/a | n/a | n/a |
INSERM
Curated by ChEMBL
| Assay Description
Agonist activity at human FXR expressed in COS1 cells by luciferase assay |
J Med Chem 51: 1831-41 (2008)
Article DOI: 10.1021/jm7015864
BindingDB Entry DOI: 10.7270/Q2222VNJ |
More data for this
Ligand-Target Pair | |
Bile acid receptor
(Homo sapiens (Human)) | BDBM50375556
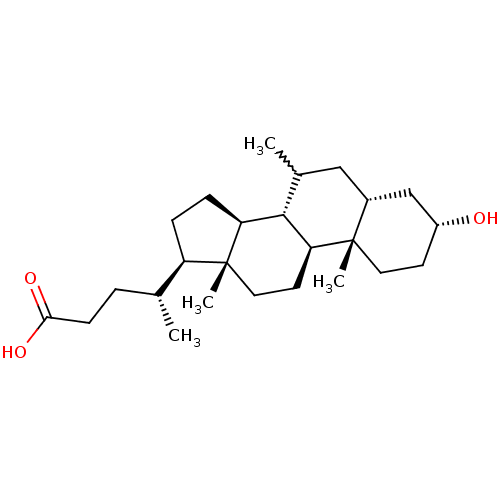
(CHEMBL270105)Show SMILES C[C@H](CCC(O)=O)[C@H]1CC[C@H]2[C@@H]3C(C)C[C@@H]4C[C@H](O)CC[C@]4(C)[C@H]3CC[C@]12C |w:12.12| Show InChI InChI=1S/C25H42O3/c1-15(5-8-22(27)28)19-6-7-20-23-16(2)13-17-14-18(26)9-11-24(17,3)21(23)10-12-25(19,20)4/h15-21,23,26H,5-14H2,1-4H3,(H,27,28)/t15-,16?,17-,18-,19-,20+,21+,23+,24+,25-/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 1.62E+4 | n/a | n/a | n/a | n/a |
INSERM
Curated by ChEMBL
| Assay Description
Agonist activity at human FXR expressed in COS1 cells by luciferase assay |
J Med Chem 51: 1831-41 (2008)
Article DOI: 10.1021/jm7015864
BindingDB Entry DOI: 10.7270/Q2222VNJ |
More data for this
Ligand-Target Pair | |
Bile acid receptor
(Homo sapiens (Human)) | BDBM53721

((4R)-4-[(3R,5S,7S,8R,9S,10S,13R,14S,17R)-10,13-dim...)Show SMILES C[C@H](CCC(O)=O)[C@H]1CC[C@H]2[C@@H]3[C@@H](O)C[C@@H]4C[C@H](O)CC[C@]4(C)[C@H]3CC[C@]12C Show InChI InChI=1S/C24H40O4/c1-14(4-7-21(27)28)17-5-6-18-22-19(9-11-24(17,18)3)23(2)10-8-16(25)12-15(23)13-20(22)26/h14-20,22,25-26H,4-13H2,1-3H3,(H,27,28)/t14-,15+,16-,17-,18+,19+,20+,22+,23+,24-/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
Article
PubMed
| n/a | n/a | n/a | n/a | >5.00E+4 | n/a | n/a | n/a | n/a |
INSERM
Curated by ChEMBL
| Assay Description
Agonist activity at human FXR expressed in COS1 cells by luciferase assay |
J Med Chem 51: 1831-41 (2008)
Article DOI: 10.1021/jm7015864
BindingDB Entry DOI: 10.7270/Q2222VNJ |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Bile acid receptor
(Homo sapiens (Human)) | BDBM21674

((4R)-4-[(1S,2S,5R,7S,9R,10R,11S,14R,15R)-5,9-dihyd...)Show SMILES [H][C@@]1(CC[C@@]2([H])[C@]3([H])[C@H](O)C[C@]4([H])C[C@H](O)CC[C@]4(C)[C@@]3([H])CC[C@]12C)[C@H](C)CCC(O)=O Show InChI InChI=1S/C24H40O4/c1-14(4-7-21(27)28)17-5-6-18-22-19(9-11-24(17,18)3)23(2)10-8-16(25)12-15(23)13-20(22)26/h14-20,22,25-26H,4-13H2,1-3H3,(H,27,28)/t14-,15+,16-,17-,18+,19+,20-,22+,23+,24-/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
DrugBank
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| DrugBank
PDB
Article
PubMed
| n/a | n/a | n/a | n/a | 1.30E+4 | n/a | n/a | n/a | n/a |
INSERM
Curated by ChEMBL
| Assay Description
Agonist activity at human FXR expressed in COS1 cells by luciferase assay |
J Med Chem 51: 1831-41 (2008)
Article DOI: 10.1021/jm7015864
BindingDB Entry DOI: 10.7270/Q2222VNJ |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Bile acid receptor
(Homo sapiens (Human)) | BDBM50375557
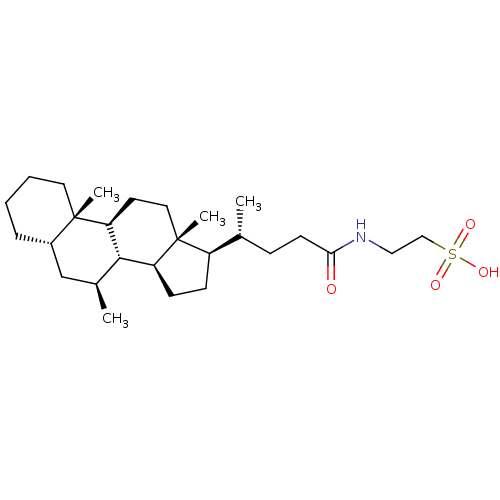
(CHEMBL261826)Show SMILES C[C@H](CCC(=O)NCCS(O)(=O)=O)[C@H]1CC[C@H]2[C@@H]3[C@@H](C)C[C@@H]4CCCC[C@]4(C)[C@H]3CC[C@]12C Show InChI InChI=1S/C27H47NO4S/c1-18(8-11-24(29)28-15-16-33(30,31)32)21-9-10-22-25-19(2)17-20-7-5-6-13-26(20,3)23(25)12-14-27(21,22)4/h18-23,25H,5-17H2,1-4H3,(H,28,29)(H,30,31,32)/t18-,19+,20+,21-,22+,23+,25+,26+,27-/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
KEGG
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 1.06E+4 | n/a | n/a | n/a | n/a |
INSERM
Curated by ChEMBL
| Assay Description
Agonist activity at human FXR expressed in COS1 cells by luciferase assay |
J Med Chem 51: 1831-41 (2008)
Article DOI: 10.1021/jm7015864
BindingDB Entry DOI: 10.7270/Q2222VNJ |
More data for this
Ligand-Target Pair | |
G-protein coupled bile acid receptor 1
(Homo sapiens (Human)) | BDBM21674

((4R)-4-[(1S,2S,5R,7S,9R,10R,11S,14R,15R)-5,9-dihyd...)Show SMILES [H][C@@]1(CC[C@@]2([H])[C@]3([H])[C@H](O)C[C@]4([H])C[C@H](O)CC[C@]4(C)[C@@]3([H])CC[C@]12C)[C@H](C)CCC(O)=O Show InChI InChI=1S/C24H40O4/c1-14(4-7-21(27)28)17-5-6-18-22-19(9-11-24(17,18)3)23(2)10-8-16(25)12-15(23)13-20(22)26/h14-20,22,25-26H,4-13H2,1-3H3,(H,27,28)/t14-,15+,16-,17-,18+,19+,20-,22+,23+,24-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
DrugBank
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 6.71E+3 | n/a | n/a | n/a | n/a |
INSERM
Curated by ChEMBL
| Assay Description
Agonist activity at human TGR5 expressed in CHO cells by luciferase assay |
J Med Chem 51: 1831-41 (2008)
Article DOI: 10.1021/jm7015864
BindingDB Entry DOI: 10.7270/Q2222VNJ |
More data for this
Ligand-Target Pair | |
G-protein coupled bile acid receptor 1
(Homo sapiens (Human)) | BDBM8885
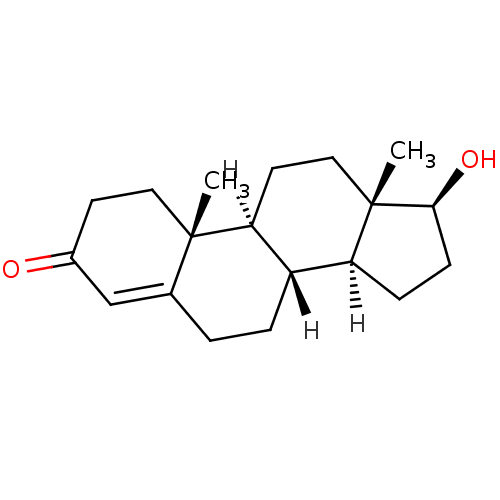
((1S,2R,10R,11S,14S,15S)-14-hydroxy-2,15-dimethylte...)Show SMILES [H][C@@]12CC[C@H](O)[C@@]1(C)CC[C@@]1([H])[C@@]2([H])CCC2=CC(=O)CC[C@]12C |r,t:18| Show InChI InChI=1S/C19H28O2/c1-18-9-7-13(20)11-12(18)3-4-14-15-5-6-17(21)19(15,2)10-8-16(14)18/h11,14-17,21H,3-10H2,1-2H3/t14-,15-,16-,17-,18-,19-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
DrugBank
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 1.83E+4 | n/a | n/a | n/a | n/a |
INSERM
Curated by ChEMBL
| Assay Description
Agonist activity at human TGR5 expressed in CHO cells by luciferase assay |
J Med Chem 51: 1831-41 (2008)
Article DOI: 10.1021/jm7015864
BindingDB Entry DOI: 10.7270/Q2222VNJ |
More data for this
Ligand-Target Pair | |
G-protein coupled bile acid receptor 1
(Homo sapiens (Human)) | BDBM18161

((1S,2S,7S,10R,11S,14S,15S)-14-hydroxy-2,15-dimethy...)Show SMILES [H][C@@]12CC[C@H](O)[C@@]1(C)CC[C@@]1([H])[C@@]2([H])CC[C@@]2([H])CC(=O)CC[C@]12C |r| Show InChI InChI=1S/C19H30O2/c1-18-9-7-13(20)11-12(18)3-4-14-15-5-6-17(21)19(15,2)10-8-16(14)18/h12,14-17,21H,3-11H2,1-2H3/t12-,14-,15-,16-,17-,18-,19-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 4.49E+3 | n/a | n/a | n/a | n/a |
INSERM
Curated by ChEMBL
| Assay Description
Agonist activity at human TGR5 expressed in CHO cells by luciferase assay |
J Med Chem 51: 1831-41 (2008)
Article DOI: 10.1021/jm7015864
BindingDB Entry DOI: 10.7270/Q2222VNJ |
More data for this
Ligand-Target Pair | |
G-protein coupled bile acid receptor 1
(Homo sapiens (Human)) | BDBM50375558
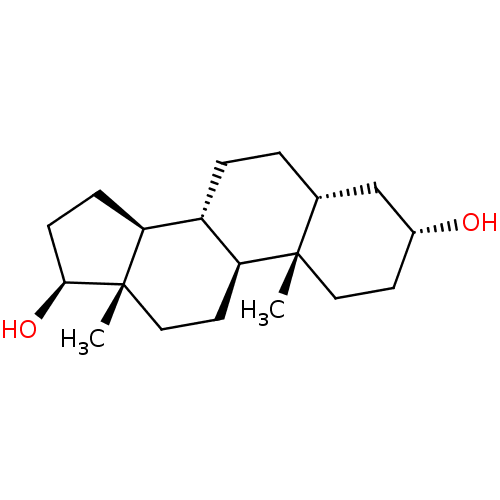
(ETIOCHOLANDIOL)Show SMILES C[C@]12CC[C@H]3[C@@H](CC[C@@H]4C[C@H](O)CC[C@]34C)[C@@H]1CC[C@@H]2O |r| Show InChI InChI=1S/C19H32O2/c1-18-9-7-13(20)11-12(18)3-4-14-15-5-6-17(21)19(15,2)10-8-16(14)18/h12-17,20-21H,3-11H2,1-2H3/t12-,13-,14+,15+,16+,17+,18+,19+/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
KEGG
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 2.44E+3 | n/a | n/a | n/a | n/a |
INSERM
Curated by ChEMBL
| Assay Description
Agonist activity at human TGR5 expressed in CHO cells by luciferase assay |
J Med Chem 51: 1831-41 (2008)
Article DOI: 10.1021/jm7015864
BindingDB Entry DOI: 10.7270/Q2222VNJ |
More data for this
Ligand-Target Pair | |
G-protein coupled bile acid receptor 1
(Homo sapiens (Human)) | BDBM50375559

(CHEMBL259898)Show SMILES C[C@]12CC[C@H]3[C@@H](CC=C4C[C@H](CC[C@]34C)OS(O)(=O)=O)[C@@H]1CCC2=O |t:7| Show InChI InChI=1S/C19H28O5S/c1-18-9-7-13(24-25(21,22)23)11-12(18)3-4-14-15-5-6-17(20)19(15,2)10-8-16(14)18/h3,13-16H,4-11H2,1-2H3,(H,21,22,23)/t13-,14-,15-,16-,18-,19-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
PC cid
PC sid
PDB
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 1.27E+5 | n/a | n/a | n/a | n/a |
INSERM
Curated by ChEMBL
| Assay Description
Agonist activity at human TGR5 expressed in CHO cells by luciferase assay |
J Med Chem 51: 1831-41 (2008)
Article DOI: 10.1021/jm7015864
BindingDB Entry DOI: 10.7270/Q2222VNJ |
More data for this
Ligand-Target Pair | |
G-protein coupled bile acid receptor 1
(Homo sapiens (Human)) | BDBM50223368

(3beta-hydroxyandrost-5-en-17-one | CHEMBL90593 | D...)Show SMILES C[C@]12CC[C@H]3[C@@H](CC=C4C[C@@H](O)CC[C@]34C)[C@@H]1CCC2=O |r,t:7| Show InChI InChI=1S/C19H28O2/c1-18-9-7-13(20)11-12(18)3-4-14-15-5-6-17(21)19(15,2)10-8-16(14)18/h3,13-16,20H,4-11H2,1-2H3/t13-,14-,15-,16-,18-,19-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 3.33E+3 | n/a | n/a | n/a | n/a |
INSERM
Curated by ChEMBL
| Assay Description
Agonist activity at human TGR5 expressed in CHO cells by luciferase assay |
J Med Chem 51: 1831-41 (2008)
Article DOI: 10.1021/jm7015864
BindingDB Entry DOI: 10.7270/Q2222VNJ |
More data for this
Ligand-Target Pair | |
G-protein coupled bile acid receptor 1
(Homo sapiens (Human)) | BDBM50375560
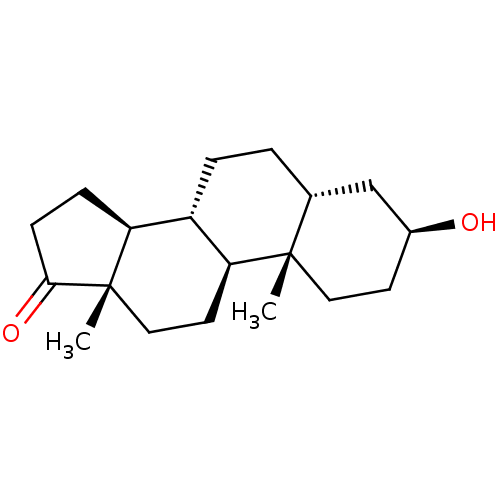
(EPIETIOCHOLANOLONE)Show SMILES C[C@]12CC[C@H]3[C@@H](CC[C@@H]4C[C@@H](O)CC[C@]34C)[C@@H]1CCC2=O Show InChI InChI=1S/C19H30O2/c1-18-9-7-13(20)11-12(18)3-4-14-15-5-6-17(21)19(15,2)10-8-16(14)18/h12-16,20H,3-11H2,1-2H3/t12-,13+,14+,15+,16+,18+,19+/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 2.62E+3 | n/a | n/a | n/a | n/a |
INSERM
Curated by ChEMBL
| Assay Description
Agonist activity at human TGR5 expressed in CHO cells by luciferase assay |
J Med Chem 51: 1831-41 (2008)
Article DOI: 10.1021/jm7015864
BindingDB Entry DOI: 10.7270/Q2222VNJ |
More data for this
Ligand-Target Pair | |
G-protein coupled bile acid receptor 1
(Homo sapiens (Human)) | BDBM50191348
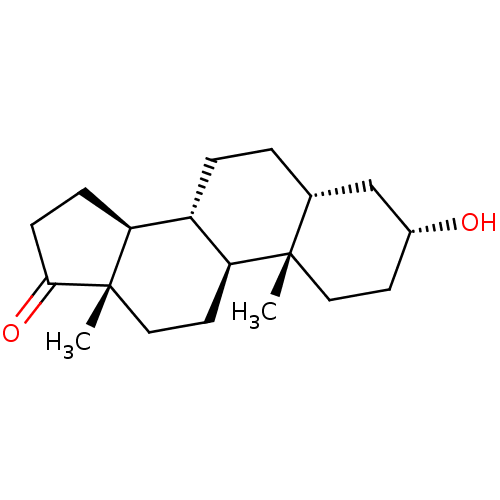
((3alpha,5beta)-3-hydroxyandrostan-17-one | 3alpha-...)Show SMILES C[C@]12CC[C@H]3[C@@H](CC[C@@H]4C[C@H](O)CC[C@]34C)[C@@H]1CCC2=O |r| Show InChI InChI=1S/C19H30O2/c1-18-9-7-13(20)11-12(18)3-4-14-15-5-6-17(21)19(15,2)10-8-16(14)18/h12-16,20H,3-11H2,1-2H3/t12-,13-,14+,15+,16+,18+,19+/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 3.76E+3 | n/a | n/a | n/a | n/a |
INSERM
Curated by ChEMBL
| Assay Description
Agonist activity at human TGR5 expressed in CHO cells by luciferase assay |
J Med Chem 51: 1831-41 (2008)
Article DOI: 10.1021/jm7015864
BindingDB Entry DOI: 10.7270/Q2222VNJ |
More data for this
Ligand-Target Pair | |
G-protein coupled bile acid receptor 1
(Homo sapiens (Human)) | BDBM50375561
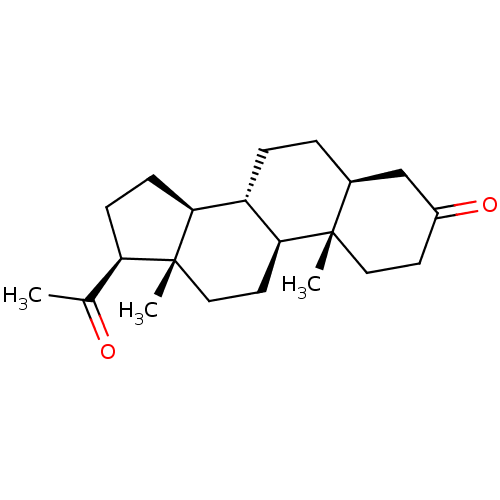
(CHEMBL407717)Show SMILES CC(=O)[C@H]1CC[C@H]2[C@@H]3CC[C@H]4CC(=O)CC[C@]4(C)[C@H]3CC[C@]12C Show InChI InChI=1S/C21H32O2/c1-13(22)17-6-7-18-16-5-4-14-12-15(23)8-10-20(14,2)19(16)9-11-21(17,18)3/h14,16-19H,4-12H2,1-3H3/t14-,16-,17+,18-,19-,20-,21+/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
KEGG
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 290 | n/a | n/a | n/a | n/a |
INSERM
Curated by ChEMBL
| Assay Description
Agonist activity at human TGR5 expressed in CHO cells by luciferase assay |
J Med Chem 51: 1831-41 (2008)
Article DOI: 10.1021/jm7015864
BindingDB Entry DOI: 10.7270/Q2222VNJ |
More data for this
Ligand-Target Pair | |
G-protein coupled bile acid receptor 1
(Homo sapiens (Human)) | BDBM17639
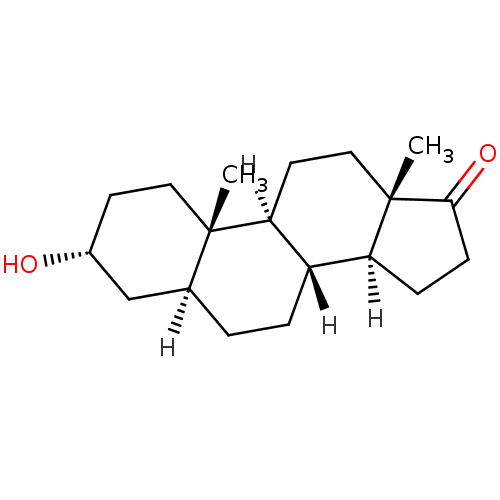
((1S,2S,5R,7S,10R,11S,15S)-5-hydroxy-2,15-dimethylt...)Show SMILES [H][C@@]12CCC(=O)[C@@]1(C)CC[C@@]1([H])[C@@]2([H])CC[C@@]2([H])C[C@H](O)CC[C@]12C Show InChI InChI=1S/C19H30O2/c1-18-9-7-13(20)11-12(18)3-4-14-15-5-6-17(21)19(15,2)10-8-16(14)18/h12-16,20H,3-11H2,1-2H3/t12-,13+,14-,15-,16-,18-,19-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 6.22E+3 | n/a | n/a | n/a | n/a |
INSERM
Curated by ChEMBL
| Assay Description
Agonist activity at human TGR5 expressed in CHO cells by luciferase assay |
J Med Chem 51: 1831-41 (2008)
Article DOI: 10.1021/jm7015864
BindingDB Entry DOI: 10.7270/Q2222VNJ |
More data for this
Ligand-Target Pair | |
G-protein coupled bile acid receptor 1
(Homo sapiens (Human)) | BDBM50375562
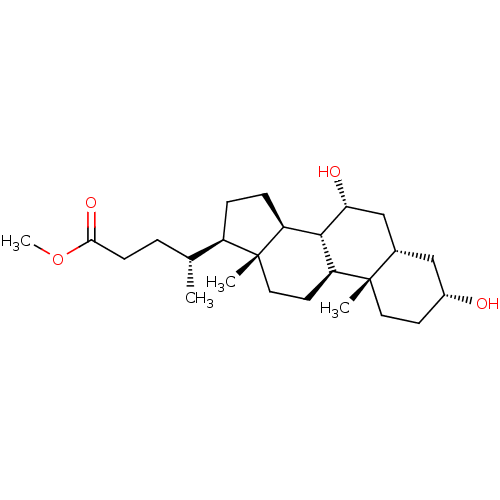
(CHEMBL273086)Show SMILES COC(=O)CC[C@@H](C)[C@H]1CC[C@H]2[C@@H]3[C@H](O)C[C@@H]4C[C@H](O)CC[C@]4(C)[C@H]3CC[C@]12C Show InChI InChI=1S/C25H42O4/c1-15(5-8-22(28)29-4)18-6-7-19-23-20(10-12-25(18,19)3)24(2)11-9-17(26)13-16(24)14-21(23)27/h15-21,23,26-27H,5-14H2,1-4H3/t15-,16+,17-,18-,19+,20+,21-,23+,24+,25-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 4.39E+3 | n/a | n/a | n/a | n/a |
INSERM
Curated by ChEMBL
| Assay Description
Agonist activity at human TGR5 expressed in CHO cells by luciferase assay |
J Med Chem 51: 1831-41 (2008)
Article DOI: 10.1021/jm7015864
BindingDB Entry DOI: 10.7270/Q2222VNJ |
More data for this
Ligand-Target Pair | |
G-protein coupled bile acid receptor 1
(Homo sapiens (Human)) | BDBM50375563
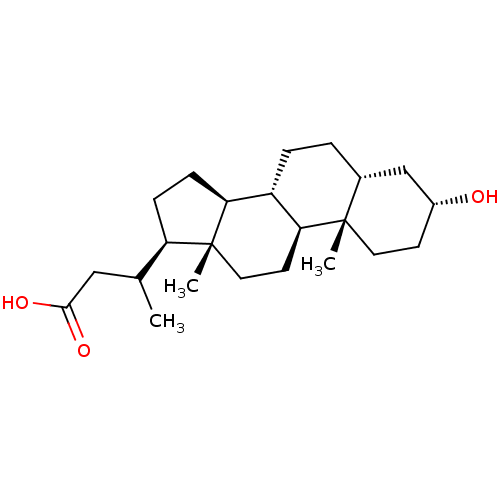
(CHEMBL160902)Show SMILES CC(CC(O)=O)[C@H]1CC[C@H]2[C@@H]3CC[C@@H]4C[C@H](O)CC[C@]4(C)[C@H]3CC[C@]12C Show InChI InChI=1S/C23H38O3/c1-14(12-21(25)26)18-6-7-19-17-5-4-15-13-16(24)8-10-22(15,2)20(17)9-11-23(18,19)3/h14-20,24H,4-13H2,1-3H3,(H,25,26)/t14?,15-,16-,17+,18-,19+,20+,22+,23-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 770 | n/a | n/a | n/a | n/a |
INSERM
Curated by ChEMBL
| Assay Description
Agonist activity at human TGR5 expressed in CHO cells by luciferase assay |
J Med Chem 51: 1831-41 (2008)
Article DOI: 10.1021/jm7015864
BindingDB Entry DOI: 10.7270/Q2222VNJ |
More data for this
Ligand-Target Pair | |
G-protein coupled bile acid receptor 1
(Homo sapiens (Human)) | BDBM50375564

(CHEMBL260735 | LCAME)Show SMILES COC(=O)CC[C@@H](C)[C@H]1CC[C@H]2[C@@H]3CC[C@@H]4C[C@H](O)CC[C@]4(C)[C@H]3CC[C@]12C Show InChI InChI=1S/C25H42O3/c1-16(5-10-23(27)28-4)20-8-9-21-19-7-6-17-15-18(26)11-13-24(17,2)22(19)12-14-25(20,21)3/h16-22,26H,5-15H2,1-4H3/t16-,17-,18-,19+,20-,21+,22+,24+,25-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 590 | n/a | n/a | n/a | n/a |
INSERM
Curated by ChEMBL
| Assay Description
Agonist activity at human TGR5 expressed in CHO cells by luciferase assay |
J Med Chem 51: 1831-41 (2008)
Article DOI: 10.1021/jm7015864
BindingDB Entry DOI: 10.7270/Q2222VNJ |
More data for this
Ligand-Target Pair | |
G-protein coupled bile acid receptor 1
(Homo sapiens (Human)) | BDBM50375565
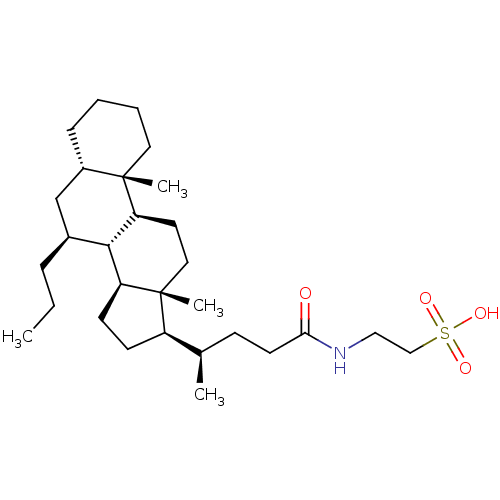
(CHEMBL260737)Show SMILES CCC[C@H]1C[C@@H]2CCCC[C@]2(C)[C@H]2CC[C@]3(C)[C@H](CC[C@H]3[C@H]12)[C@H](C)CCC(=O)NCCS(O)(=O)=O Show InChI InChI=1S/C29H51NO4S/c1-5-8-21-19-22-9-6-7-15-28(22,3)25-14-16-29(4)23(11-12-24(29)27(21)25)20(2)10-13-26(31)30-17-18-35(32,33)34/h20-25,27H,5-19H2,1-4H3,(H,30,31)(H,32,33,34)/t20-,21+,22+,23-,24+,25+,27+,28+,29-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
KEGG
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 360 | n/a | n/a | n/a | n/a |
INSERM
Curated by ChEMBL
| Assay Description
Agonist activity at human TGR5 expressed in CHO cells by luciferase assay |
J Med Chem 51: 1831-41 (2008)
Article DOI: 10.1021/jm7015864
BindingDB Entry DOI: 10.7270/Q2222VNJ |
More data for this
Ligand-Target Pair | |
G-protein coupled bile acid receptor 1
(Homo sapiens (Human)) | BDBM26270
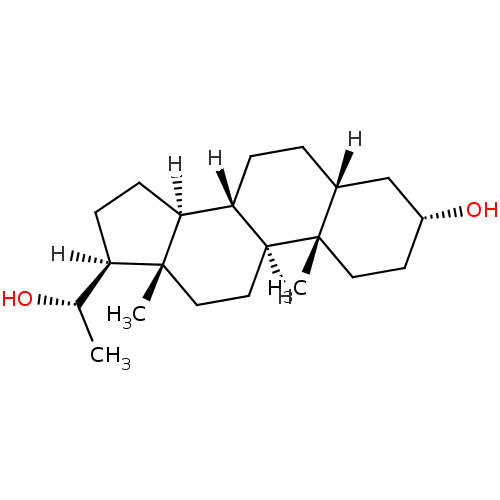
((1S,2S,5R,7R,10R,11S,14S,15S)-14-[(1S)-1-hydroxyet...)Show SMILES [H][C@@]1(CC[C@@]2([H])[C@]3([H])CC[C@]4([H])C[C@H](O)CC[C@]4(C)[C@@]3([H])CC[C@]12C)[C@H](C)O Show InChI InChI=1S/C21H36O2/c1-13(22)17-6-7-18-16-5-4-14-12-15(23)8-10-20(14,2)19(16)9-11-21(17,18)3/h13-19,22-23H,4-12H2,1-3H3/t13-,14+,15+,16-,17+,18-,19-,20-,21+/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
KEGG
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 850 | n/a | n/a | n/a | n/a |
INSERM
Curated by ChEMBL
| Assay Description
Agonist activity at human TGR5 expressed in CHO cells by luciferase assay |
J Med Chem 51: 1831-41 (2008)
Article DOI: 10.1021/jm7015864
BindingDB Entry DOI: 10.7270/Q2222VNJ |
More data for this
Ligand-Target Pair | |
G-protein coupled bile acid receptor 1
(Homo sapiens (Human)) | BDBM50375549

(CHEMBL1628228)Show SMILES C[C@H](CCCS(O)(=O)=O)[C@H]1CCC2C3[C@@H](O)C[C@@H]4C[C@H](O)CC[C@]4(C)C3CC[C@]12C |r| Show InChI InChI=1S/C24H42O5S/c1-15(5-4-12-30(27,28)29)18-6-7-19-22-20(9-11-24(18,19)3)23(2)10-8-17(25)13-16(23)14-21(22)26/h15-22,25-26H,4-14H2,1-3H3,(H,27,28,29)/t15-,16+,17-,18-,19?,20?,21+,22?,23+,24-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
KEGG
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 5.02E+3 | n/a | n/a | n/a | n/a |
INSERM
Curated by ChEMBL
| Assay Description
Agonist activity at human TGR5 expressed in CHO cells by luciferase assay |
J Med Chem 51: 1831-41 (2008)
Article DOI: 10.1021/jm7015864
BindingDB Entry DOI: 10.7270/Q2222VNJ |
More data for this
Ligand-Target Pair | |
G-protein coupled bile acid receptor 1
(Homo sapiens (Human)) | BDBM50334959
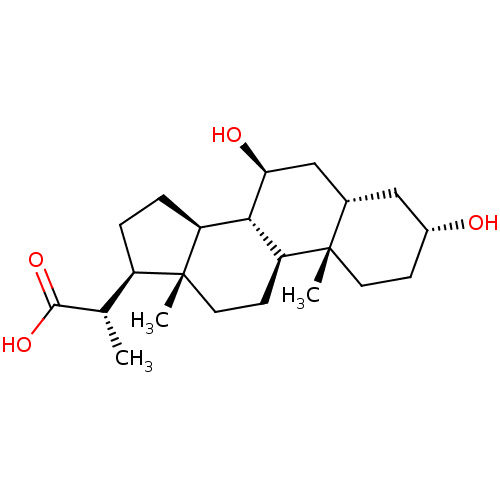
((S)-2-((3R,5S,7S,8R,9S,10S,13S,14S,17R)-3,7-dihydr...)Show SMILES C[C@@H]([C@H]1CC[C@H]2[C@@H]3[C@@H](O)C[C@@H]4C[C@H](O)CC[C@]4(C)[C@H]3CC[C@]12C)C(O)=O |r| Show InChI InChI=1S/C22H36O4/c1-12(20(25)26)15-4-5-16-19-17(7-9-22(15,16)3)21(2)8-6-14(23)10-13(21)11-18(19)24/h12-19,23-24H,4-11H2,1-3H3,(H,25,26)/t12-,13-,14+,15+,16-,17-,18-,19-,21-,22+/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | >3.16E+5 | n/a | n/a | n/a | n/a |
INSERM
Curated by ChEMBL
| Assay Description
Agonist activity at human TGR5 expressed in CHO cells by luciferase assay |
J Med Chem 51: 1831-41 (2008)
Article DOI: 10.1021/jm7015864
BindingDB Entry DOI: 10.7270/Q2222VNJ |
More data for this
Ligand-Target Pair | |
G-protein coupled bile acid receptor 1
(Homo sapiens (Human)) | BDBM50375566
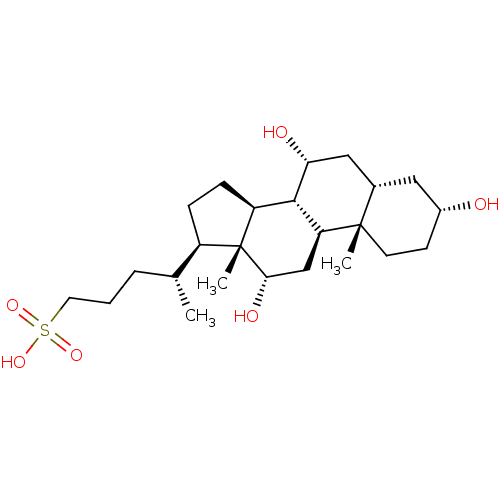
(CHEMBL408445)Show SMILES C[C@H](CCCS(O)(=O)=O)[C@H]1CC[C@H]2[C@@H]3[C@H](O)C[C@@H]4C[C@H](O)CC[C@]4(C)[C@H]3C[C@H](O)[C@]12C Show InChI InChI=1S/C24H42O6S/c1-14(5-4-10-31(28,29)30)17-6-7-18-22-19(13-21(27)24(17,18)3)23(2)9-8-16(25)11-15(23)12-20(22)26/h14-22,25-27H,4-13H2,1-3H3,(H,28,29,30)/t14-,15+,16-,17-,18+,19+,20-,21+,22+,23+,24-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
KEGG
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 1.00E+3 | n/a | n/a | n/a | n/a |
INSERM
Curated by ChEMBL
| Assay Description
Agonist activity at human TGR5 expressed in CHO cells by luciferase assay |
J Med Chem 51: 1831-41 (2008)
Article DOI: 10.1021/jm7015864
BindingDB Entry DOI: 10.7270/Q2222VNJ |
More data for this
Ligand-Target Pair | |
G-protein coupled bile acid receptor 1
(Homo sapiens (Human)) | BDBM50375567
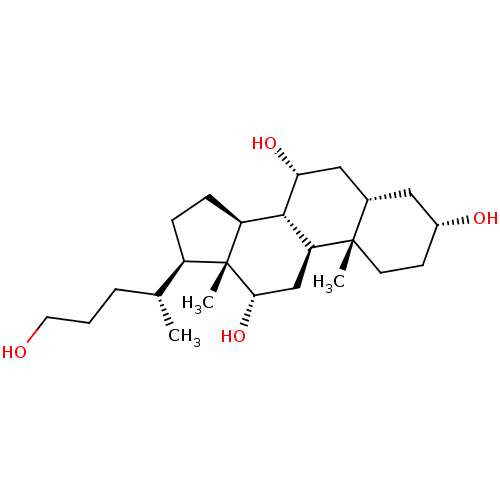
(CHEMBL270703)Show SMILES C[C@H](CCCO)[C@H]1CC[C@H]2[C@@H]3[C@H](O)C[C@@H]4C[C@H](O)CC[C@]4(C)[C@H]3C[C@H](O)[C@]12C Show InChI InChI=1S/C24H42O4/c1-14(5-4-10-25)17-6-7-18-22-19(13-21(28)24(17,18)3)23(2)9-8-16(26)11-15(23)12-20(22)27/h14-22,25-28H,4-13H2,1-3H3/t14-,15+,16-,17-,18+,19+,20-,21+,22+,23+,24-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 870 | n/a | n/a | n/a | n/a |
INSERM
Curated by ChEMBL
| Assay Description
Agonist activity at human TGR5 expressed in CHO cells by luciferase assay |
J Med Chem 51: 1831-41 (2008)
Article DOI: 10.1021/jm7015864
BindingDB Entry DOI: 10.7270/Q2222VNJ |
More data for this
Ligand-Target Pair | |
G-protein coupled bile acid receptor 1
(Homo sapiens (Human)) | BDBM50375568
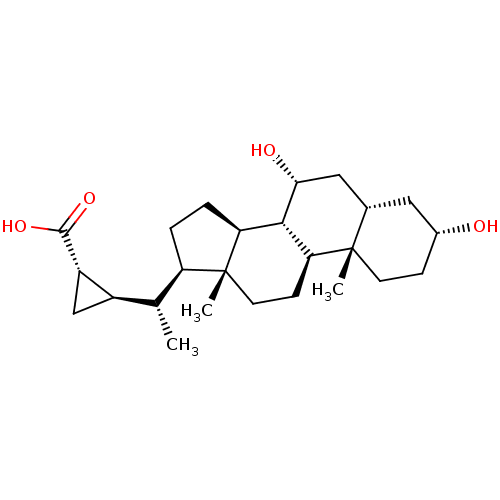
(CHEMBL270912)Show SMILES C[C@H]([C@H]1C[C@@H]1C(O)=O)[C@H]1CC[C@H]2[C@@H]3[C@H](O)C[C@@H]4C[C@H](O)CC[C@]4(C)[C@H]3CC[C@]12C Show InChI InChI=1S/C25H40O4/c1-13(16-12-17(16)23(28)29)18-4-5-19-22-20(7-9-25(18,19)3)24(2)8-6-15(26)10-14(24)11-21(22)27/h13-22,26-27H,4-12H2,1-3H3,(H,28,29)/t13-,14+,15-,16-,17+,18-,19+,20+,21-,22+,24+,25-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | >1.00E+5 | n/a | n/a | n/a | n/a |
INSERM
Curated by ChEMBL
| Assay Description
Agonist activity at human TGR5 expressed in CHO cells by luciferase assay |
J Med Chem 51: 1831-41 (2008)
Article DOI: 10.1021/jm7015864
BindingDB Entry DOI: 10.7270/Q2222VNJ |
More data for this
Ligand-Target Pair | |
G-protein coupled bile acid receptor 1
(Homo sapiens (Human)) | BDBM50375569
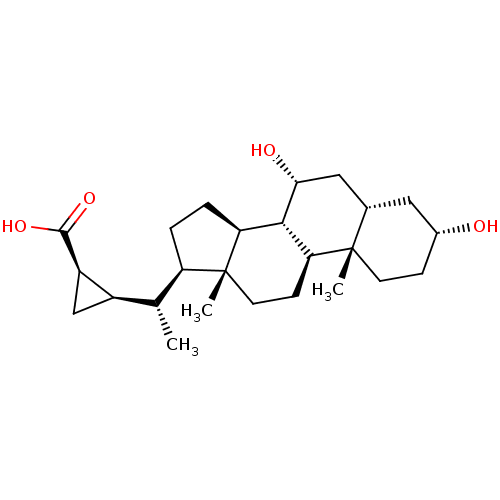
(CHEMBL409510)Show SMILES C[C@H]([C@H]1C[C@H]1C(O)=O)[C@H]1CC[C@H]2[C@@H]3[C@H](O)C[C@@H]4C[C@H](O)CC[C@]4(C)[C@H]3CC[C@]12C Show InChI InChI=1S/C25H40O4/c1-13(16-12-17(16)23(28)29)18-4-5-19-22-20(7-9-25(18,19)3)24(2)8-6-15(26)10-14(24)11-21(22)27/h13-22,26-27H,4-12H2,1-3H3,(H,28,29)/t13-,14+,15-,16-,17-,18-,19+,20+,21-,22+,24+,25-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 7.57E+4 | n/a | n/a | n/a | n/a |
INSERM
Curated by ChEMBL
| Assay Description
Agonist activity at human TGR5 expressed in CHO cells by luciferase assay |
J Med Chem 51: 1831-41 (2008)
Article DOI: 10.1021/jm7015864
BindingDB Entry DOI: 10.7270/Q2222VNJ |
More data for this
Ligand-Target Pair | |
G-protein coupled bile acid receptor 1
(Homo sapiens (Human)) | BDBM50375570
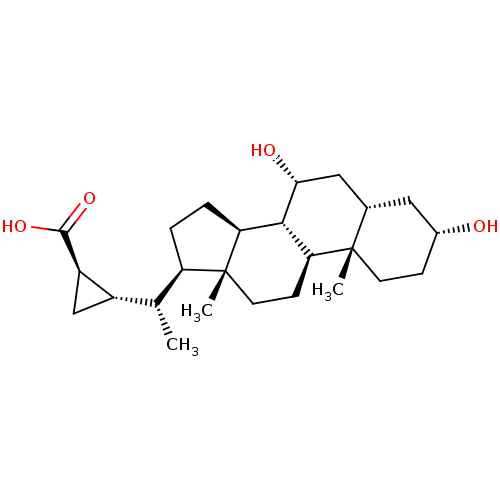
(CHEMBL272426)Show SMILES C[C@H]([C@@H]1C[C@H]1C(O)=O)[C@H]1CC[C@H]2[C@@H]3[C@H](O)C[C@@H]4C[C@H](O)CC[C@]4(C)[C@H]3CC[C@]12C Show InChI InChI=1S/C25H40O4/c1-13(16-12-17(16)23(28)29)18-4-5-19-22-20(7-9-25(18,19)3)24(2)8-6-15(26)10-14(24)11-21(22)27/h13-22,26-27H,4-12H2,1-3H3,(H,28,29)/t13-,14+,15-,16+,17-,18-,19+,20+,21-,22+,24+,25-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 2.91E+3 | n/a | n/a | n/a | n/a |
INSERM
Curated by ChEMBL
| Assay Description
Agonist activity at human TGR5 expressed in CHO cells by luciferase assay |
J Med Chem 51: 1831-41 (2008)
Article DOI: 10.1021/jm7015864
BindingDB Entry DOI: 10.7270/Q2222VNJ |
More data for this
Ligand-Target Pair | |
G-protein coupled bile acid receptor 1
(Homo sapiens (Human)) | BDBM50375571
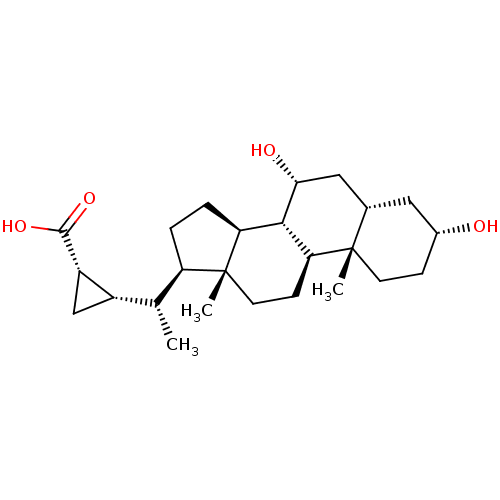
(CHEMBL409511)Show SMILES C[C@H]([C@@H]1C[C@@H]1C(O)=O)[C@H]1CC[C@H]2[C@@H]3[C@H](O)C[C@@H]4C[C@H](O)CC[C@]4(C)[C@H]3CC[C@]12C Show InChI InChI=1S/C25H40O4/c1-13(16-12-17(16)23(28)29)18-4-5-19-22-20(7-9-25(18,19)3)24(2)8-6-15(26)10-14(24)11-21(22)27/h13-22,26-27H,4-12H2,1-3H3,(H,28,29)/t13-,14+,15-,16+,17+,18-,19+,20+,21-,22+,24+,25-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 1.33E+3 | n/a | n/a | n/a | n/a |
INSERM
Curated by ChEMBL
| Assay Description
Agonist activity at human TGR5 expressed in CHO cells by luciferase assay |
J Med Chem 51: 1831-41 (2008)
Article DOI: 10.1021/jm7015864
BindingDB Entry DOI: 10.7270/Q2222VNJ |
More data for this
Ligand-Target Pair | |
G-protein coupled bile acid receptor 1
(Homo sapiens (Human)) | BDBM50375551

(CHEMBL406923)Show SMILES C[C@H](CCCS(O)(=O)=O)[C@H]1CC[C@H]2[C@@H]3[C@H](O)C[C@@H]4C[C@H](O)CC[C@]4(C)[C@H]3CC[C@]12C Show InChI InChI=1S/C24H42O5S/c1-15(5-4-12-30(27,28)29)18-6-7-19-22-20(9-11-24(18,19)3)23(2)10-8-17(25)13-16(23)14-21(22)26/h15-22,25-26H,4-14H2,1-3H3,(H,27,28,29)/t15-,16+,17-,18-,19+,20+,21-,22+,23+,24-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
KEGG
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 440 | n/a | n/a | n/a | n/a |
INSERM
Curated by ChEMBL
| Assay Description
Agonist activity at human TGR5 expressed in CHO cells by luciferase assay |
J Med Chem 51: 1831-41 (2008)
Article DOI: 10.1021/jm7015864
BindingDB Entry DOI: 10.7270/Q2222VNJ |
More data for this
Ligand-Target Pair | |
G-protein coupled bile acid receptor 1
(Homo sapiens (Human)) | BDBM50375552

(CHEMBL270287)Show SMILES C[C@H](CCCO)[C@H]1CC[C@H]2[C@@H]3[C@H](O)C[C@@H]4C[C@H](O)CC[C@]4(C)[C@H]3CC[C@]12C Show InChI InChI=1S/C24H42O3/c1-15(5-4-12-25)18-6-7-19-22-20(9-11-24(18,19)3)23(2)10-8-17(26)13-16(23)14-21(22)27/h15-22,25-27H,4-14H2,1-3H3/t15-,16+,17-,18-,19+,20+,21-,22+,23+,24-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 120 | n/a | n/a | n/a | n/a |
INSERM
Curated by ChEMBL
| Assay Description
Agonist activity at human TGR5 expressed in CHO cells by luciferase assay |
J Med Chem 51: 1831-41 (2008)
Article DOI: 10.1021/jm7015864
BindingDB Entry DOI: 10.7270/Q2222VNJ |
More data for this
Ligand-Target Pair | |
G-protein coupled bile acid receptor 1
(Homo sapiens (Human)) | BDBM50375572
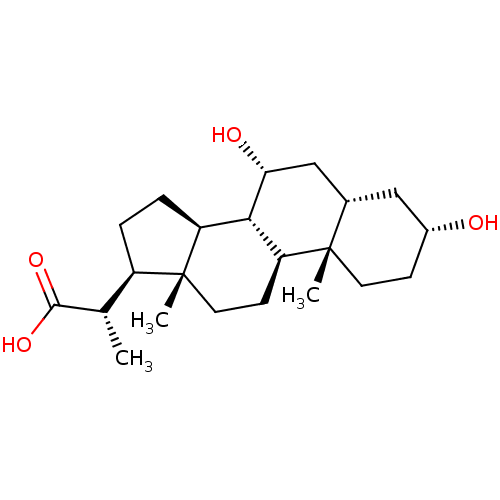
(CHEMBL272409)Show SMILES C[C@@H]([C@H]1CC[C@H]2[C@@H]3[C@H](O)C[C@@H]4C[C@H](O)CC[C@]4(C)[C@H]3CC[C@]12C)C(O)=O Show InChI InChI=1S/C22H36O4/c1-12(20(25)26)15-4-5-16-19-17(7-9-22(15,16)3)21(2)8-6-14(23)10-13(21)11-18(19)24/h12-19,23-24H,4-11H2,1-3H3,(H,25,26)/t12-,13-,14+,15+,16-,17-,18+,19-,21-,22+/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | >1.00E+5 | n/a | n/a | n/a | n/a |
INSERM
Curated by ChEMBL
| Assay Description
Agonist activity at human TGR5 expressed in CHO cells by luciferase assay |
J Med Chem 51: 1831-41 (2008)
Article DOI: 10.1021/jm7015864
BindingDB Entry DOI: 10.7270/Q2222VNJ |
More data for this
Ligand-Target Pair | |
G-protein coupled bile acid receptor 1
(Homo sapiens (Human)) | BDBM50375573
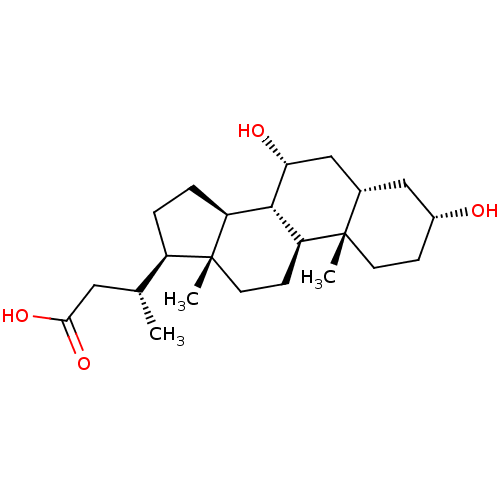
(CHEMBL408175)Show SMILES C[C@H](CC(O)=O)[C@H]1CC[C@H]2[C@@H]3[C@H](O)C[C@@H]4C[C@H](O)CC[C@]4(C)[C@H]3CC[C@]12C Show InChI InChI=1S/C23H38O4/c1-13(10-20(26)27)16-4-5-17-21-18(7-9-23(16,17)3)22(2)8-6-15(24)11-14(22)12-19(21)25/h13-19,21,24-25H,4-12H2,1-3H3,(H,26,27)/t13-,14+,15-,16-,17+,18+,19-,21+,22+,23-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 1.04E+4 | n/a | n/a | n/a | n/a |
INSERM
Curated by ChEMBL
| Assay Description
Agonist activity at human TGR5 expressed in CHO cells by luciferase assay |
J Med Chem 51: 1831-41 (2008)
Article DOI: 10.1021/jm7015864
BindingDB Entry DOI: 10.7270/Q2222VNJ |
More data for this
Ligand-Target Pair | |
G-protein coupled bile acid receptor 1
(Homo sapiens (Human)) | BDBM50375574
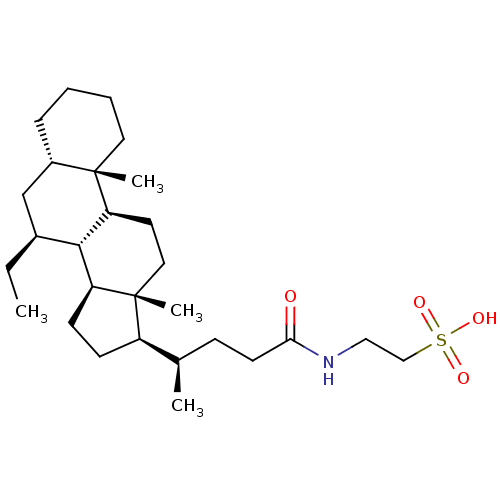
(CHEMBL408477)Show SMILES CC[C@H]1C[C@@H]2CCCC[C@]2(C)[C@H]2CC[C@]3(C)[C@H](CC[C@H]3[C@H]12)[C@H](C)CCC(=O)NCCS(O)(=O)=O Show InChI InChI=1S/C28H49NO4S/c1-5-20-18-21-8-6-7-14-27(21,3)24-13-15-28(4)22(10-11-23(28)26(20)24)19(2)9-12-25(30)29-16-17-34(31,32)33/h19-24,26H,5-18H2,1-4H3,(H,29,30)(H,31,32,33)/t19-,20+,21+,22-,23+,24+,26+,27+,28-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
KEGG
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 730 | n/a | n/a | n/a | n/a |
INSERM
Curated by ChEMBL
| Assay Description
Agonist activity at human TGR5 expressed in CHO cells by luciferase assay |
J Med Chem 51: 1831-41 (2008)
Article DOI: 10.1021/jm7015864
BindingDB Entry DOI: 10.7270/Q2222VNJ |
More data for this
Ligand-Target Pair | |
G-protein coupled bile acid receptor 1
(Homo sapiens (Human)) | BDBM50375557

(CHEMBL261826)Show SMILES C[C@H](CCC(=O)NCCS(O)(=O)=O)[C@H]1CC[C@H]2[C@@H]3[C@@H](C)C[C@@H]4CCCC[C@]4(C)[C@H]3CC[C@]12C Show InChI InChI=1S/C27H47NO4S/c1-18(8-11-24(29)28-15-16-33(30,31)32)21-9-10-22-25-19(2)17-20-7-5-6-13-26(20,3)23(25)12-14-27(21,22)4/h18-23,25H,5-17H2,1-4H3,(H,28,29)(H,30,31,32)/t18-,19+,20+,21-,22+,23+,25+,26+,27-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
KEGG
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 2.36E+3 | n/a | n/a | n/a | n/a |
INSERM
Curated by ChEMBL
| Assay Description
Agonist activity at human TGR5 expressed in CHO cells by luciferase assay |
J Med Chem 51: 1831-41 (2008)
Article DOI: 10.1021/jm7015864
BindingDB Entry DOI: 10.7270/Q2222VNJ |
More data for this
Ligand-Target Pair | |
G-protein coupled bile acid receptor 1
(Homo sapiens (Human)) | BDBM50375575
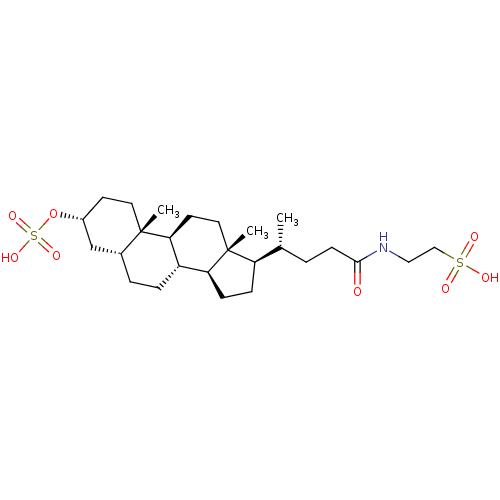
(CHEMBL270493)Show SMILES C[C@H](CCC(=O)NCCS(O)(=O)=O)[C@H]1CC[C@H]2[C@@H]3CC[C@@H]4C[C@@H](CC[C@]4(C)[C@H]3CC[C@]12C)OS(O)(=O)=O Show InChI InChI=1S/C26H45NO8S2/c1-17(4-9-24(28)27-14-15-36(29,30)31)21-7-8-22-20-6-5-18-16-19(35-37(32,33)34)10-12-25(18,2)23(20)11-13-26(21,22)3/h17-23H,4-16H2,1-3H3,(H,27,28)(H,29,30,31)(H,32,33,34)/t17-,18-,19-,20+,21-,22+,23+,25+,26-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
KEGG
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 4.78E+4 | n/a | n/a | n/a | n/a |
INSERM
Curated by ChEMBL
| Assay Description
Agonist activity at human TGR5 expressed in CHO cells by luciferase assay |
J Med Chem 51: 1831-41 (2008)
Article DOI: 10.1021/jm7015864
BindingDB Entry DOI: 10.7270/Q2222VNJ |
More data for this
Ligand-Target Pair | |
G-protein coupled bile acid receptor 1
(Homo sapiens (Human)) | BDBM50375553

(CHEMBL405879)Show SMILES CCC[C@H]1C[C@@H]2CCCC[C@]2(C)[C@H]2CC[C@]3(C)[C@H](CC[C@H]3[C@H]12)[C@H](C)CCC(O)=O Show InChI InChI=1S/C27H46O2/c1-5-8-19-17-20-9-6-7-15-26(20,3)23-14-16-27(4)21(11-12-22(27)25(19)23)18(2)10-13-24(28)29/h18-23,25H,5-17H2,1-4H3,(H,28,29)/t18-,19+,20+,21-,22+,23+,25+,26+,27-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 780 | n/a | n/a | n/a | n/a |
INSERM
Curated by ChEMBL
| Assay Description
Agonist activity at human TGR5 expressed in CHO cells by luciferase assay |
J Med Chem 51: 1831-41 (2008)
Article DOI: 10.1021/jm7015864
BindingDB Entry DOI: 10.7270/Q2222VNJ |
More data for this
Ligand-Target Pair | |
G-protein coupled bile acid receptor 1
(Homo sapiens (Human)) | BDBM50375554

(CHEMBL270494)Show SMILES CC[C@H]1C[C@@H]2CCCC[C@]2(C)[C@H]2CC[C@]3(C)[C@H](CC[C@H]3[C@H]12)[C@H](C)CCC(O)=O Show InChI InChI=1S/C26H44O2/c1-5-18-16-19-8-6-7-14-25(19,3)22-13-15-26(4)20(10-11-21(26)24(18)22)17(2)9-12-23(27)28/h17-22,24H,5-16H2,1-4H3,(H,27,28)/t17-,18+,19+,20-,21+,22+,24+,25+,26-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 2.63E+3 | n/a | n/a | n/a | n/a |
INSERM
Curated by ChEMBL
| Assay Description
Agonist activity at human TGR5 expressed in CHO cells by luciferase assay |
J Med Chem 51: 1831-41 (2008)
Article DOI: 10.1021/jm7015864
BindingDB Entry DOI: 10.7270/Q2222VNJ |
More data for this
Ligand-Target Pair | |
G-protein coupled bile acid receptor 1
(Homo sapiens (Human)) | BDBM50375576
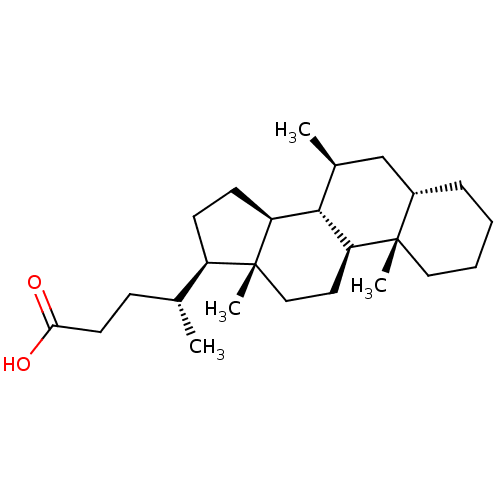
(CHEMBL407713)Show SMILES C[C@H](CCC(O)=O)[C@H]1CC[C@H]2[C@@H]3[C@@H](C)C[C@@H]4CCCC[C@]4(C)[C@H]3CC[C@]12C Show InChI InChI=1S/C25H42O2/c1-16(8-11-22(26)27)19-9-10-20-23-17(2)15-18-7-5-6-13-24(18,3)21(23)12-14-25(19,20)4/h16-21,23H,5-15H2,1-4H3,(H,26,27)/t16-,17+,18+,19-,20+,21+,23+,24+,25-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 6.18E+3 | n/a | n/a | n/a | n/a |
INSERM
Curated by ChEMBL
| Assay Description
Agonist activity at human TGR5 expressed in CHO cells by luciferase assay |
J Med Chem 51: 1831-41 (2008)
Article DOI: 10.1021/jm7015864
BindingDB Entry DOI: 10.7270/Q2222VNJ |
More data for this
Ligand-Target Pair | |
G-protein coupled bile acid receptor 1
(Homo sapiens (Human)) | BDBM50375577
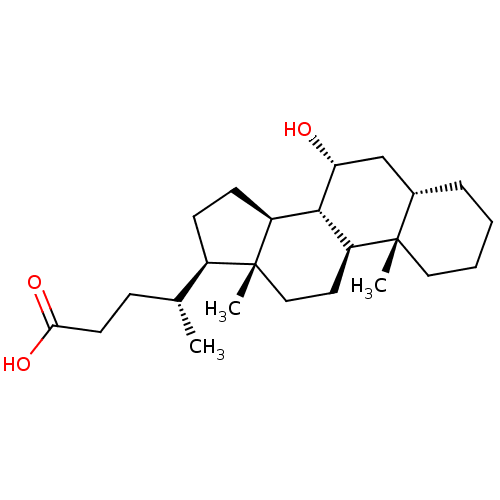
(CHEMBL270311)Show SMILES C[C@H](CCC(O)=O)[C@H]1CC[C@H]2[C@@H]3[C@H](O)C[C@@H]4CCCC[C@]4(C)[C@H]3CC[C@]12C Show InChI InChI=1S/C24H40O3/c1-15(7-10-21(26)27)17-8-9-18-22-19(11-13-24(17,18)3)23(2)12-5-4-6-16(23)14-20(22)25/h15-20,22,25H,4-14H2,1-3H3,(H,26,27)/t15-,16+,17-,18+,19+,20-,22+,23+,24-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
MMDB
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 1.45E+4 | n/a | n/a | n/a | n/a |
INSERM
Curated by ChEMBL
| Assay Description
Agonist activity at human TGR5 expressed in CHO cells by luciferase assay |
J Med Chem 51: 1831-41 (2008)
Article DOI: 10.1021/jm7015864
BindingDB Entry DOI: 10.7270/Q2222VNJ |
More data for this
Ligand-Target Pair | |
G-protein coupled bile acid receptor 1
(Homo sapiens (Human)) | BDBM50375578
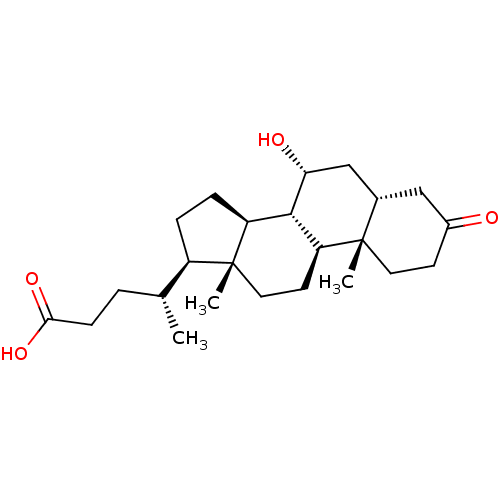
(CHEMBL271172)Show SMILES C[C@H](CCC(O)=O)[C@H]1CC[C@H]2[C@@H]3[C@H](O)C[C@@H]4CC(=O)CC[C@]4(C)[C@H]3CC[C@]12C Show InChI InChI=1S/C24H38O4/c1-14(4-7-21(27)28)17-5-6-18-22-19(9-11-24(17,18)3)23(2)10-8-16(25)12-15(23)13-20(22)26/h14-15,17-20,22,26H,4-13H2,1-3H3,(H,27,28)/t14-,15+,17-,18+,19+,20-,22+,23+,24-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 3.98E+3 | n/a | n/a | n/a | n/a |
INSERM
Curated by ChEMBL
| Assay Description
Agonist activity at human TGR5 expressed in CHO cells by luciferase assay |
J Med Chem 51: 1831-41 (2008)
Article DOI: 10.1021/jm7015864
BindingDB Entry DOI: 10.7270/Q2222VNJ |
More data for this
Ligand-Target Pair | |
G-protein coupled bile acid receptor 1
(Homo sapiens (Human)) | BDBM50375579
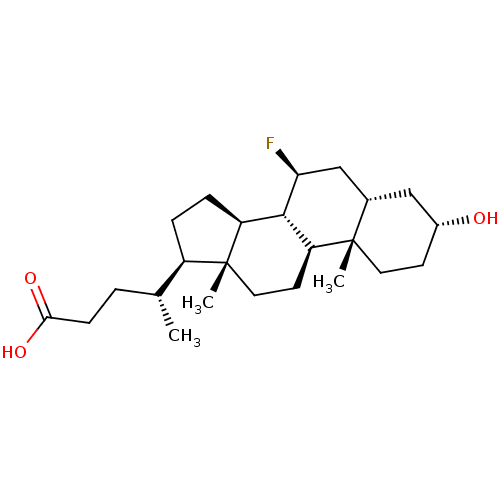
(CHEMBL260314)Show SMILES C[C@H](CCC(O)=O)[C@H]1CC[C@H]2[C@@H]3[C@@H](F)C[C@@H]4C[C@H](O)CC[C@]4(C)[C@H]3CC[C@]12C Show InChI InChI=1S/C24H39FO3/c1-14(4-7-21(27)28)17-5-6-18-22-19(9-11-24(17,18)3)23(2)10-8-16(26)12-15(23)13-20(22)25/h14-20,22,26H,4-13H2,1-3H3,(H,27,28)/t14-,15+,16-,17-,18+,19+,20+,22+,23+,24-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 2.29E+3 | n/a | n/a | n/a | n/a |
INSERM
Curated by ChEMBL
| Assay Description
Agonist activity at human TGR5 expressed in CHO cells by luciferase assay |
J Med Chem 51: 1831-41 (2008)
Article DOI: 10.1021/jm7015864
BindingDB Entry DOI: 10.7270/Q2222VNJ |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data