 Found 14 hits Enz. Inhib. hit(s) with all data for entry = 50039739
Found 14 hits Enz. Inhib. hit(s) with all data for entry = 50039739 Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Protein-tyrosine kinase 2-beta
(Homo sapiens (Human)) | BDBM50341636

((3Z)-N-(3-CHLOROPHENYL)-3-({3,5-DIMETHYL-4-[(4-MET...)Show SMILES CN(c1cccc(Cl)c1)S(=O)(=O)c1ccc2NC(=O)\C(=C/c3[nH]c(C)c(C(=O)N4CCN(C)CC4)c3C)c2c1 Show InChI InChI=1S/C28H30ClN5O4S/c1-17-25(30-18(2)26(17)28(36)34-12-10-32(3)11-13-34)16-23-22-15-21(8-9-24(22)31-27(23)35)39(37,38)33(4)20-7-5-6-19(29)14-20/h5-9,14-16,30H,10-13H2,1-4H3,(H,31,35)/b23-16- | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| 10.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Sandia National Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant PYK2 by ESI-MS analysis |
J Med Chem 55: 1926-39 (2012)
Article DOI: 10.1021/jm200979x
BindingDB Entry DOI: 10.7270/Q2R212DQ |
More data for this
Ligand-Target Pair | |
Protein-tyrosine kinase 2-beta
(Homo sapiens (Human)) | BDBM4339

((3,4-Dihydroxybenzylidene)malononitrile | 2-[(3,4-...)Show InChI InChI=1S/C10H6N2O2/c11-5-8(6-12)3-7-1-2-9(13)10(14)4-7/h1-4,13-14H | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 162 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Sandia National Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant PYK2 by ESI-MS analysis |
J Med Chem 55: 1926-39 (2012)
Article DOI: 10.1021/jm200979x
BindingDB Entry DOI: 10.7270/Q2R212DQ |
More data for this
Ligand-Target Pair | |
Protein-tyrosine kinase 2-beta
(Homo sapiens (Human)) | BDBM7364

(6-phenyl[5H]pyrrolo[2,3-b]pyrazine (aloisine) 38 |...)Show InChI InChI=1S/C17H19N3O/c1-3-4-5-14-15(12-6-8-13(21-2)9-7-12)20-17-16(14)18-10-11-19-17/h6-11H,3-5H2,1-2H3,(H,19,20) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 933 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Sandia National Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant PYK2 by ESI-MS analysis |
J Med Chem 55: 1926-39 (2012)
Article DOI: 10.1021/jm200979x
BindingDB Entry DOI: 10.7270/Q2R212DQ |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase ZAP-70
(Homo sapiens (Human)) | BDBM50383081
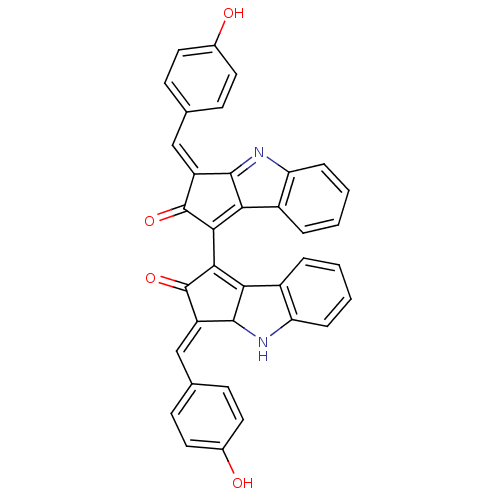
(SCYTONEMIN)Show SMILES Oc1ccc(\C=C2/C3Nc4ccccc4C3=C(C2=O)C2=C3C(=Nc4ccccc34)\C(=C/c3ccc(O)cc3)C2=O)cc1 |c:17,24,t:22| Show InChI InChI=1S/C36H22N2O4/c39-21-13-9-19(10-14-21)17-25-33-29(23-5-1-3-7-27(23)37-33)31(35(25)41)32-30-24-6-2-4-8-28(24)38-34(30)26(36(32)42)18-20-11-15-22(40)16-12-20/h1-18,33,37,39-40H/b25-17+,26-18+ | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 1.55E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Sandia National Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant ZAP70 using EEEEYEEEE as substrate by ESI-MS analysis |
J Med Chem 55: 1926-39 (2012)
Article DOI: 10.1021/jm200979x
BindingDB Entry DOI: 10.7270/Q2R212DQ |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase ZAP-70
(Homo sapiens (Human)) | BDBM7364

(6-phenyl[5H]pyrrolo[2,3-b]pyrazine (aloisine) 38 |...)Show InChI InChI=1S/C17H19N3O/c1-3-4-5-14-15(12-6-8-13(21-2)9-7-12)20-17-16(14)18-10-11-19-17/h6-11H,3-5H2,1-2H3,(H,19,20) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 2.63E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Sandia National Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant ZAP70 using EEEEYEEEE as substrate by ESI-MS analysis |
J Med Chem 55: 1926-39 (2012)
Article DOI: 10.1021/jm200979x
BindingDB Entry DOI: 10.7270/Q2R212DQ |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase ZAP-70
(Homo sapiens (Human)) | BDBM92422
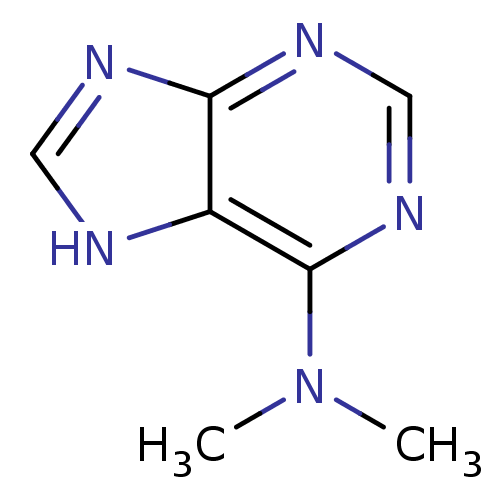
(CHEMBL407391 | PU09)Show InChI InChI=1S/C7H9N5/c1-12(2)7-5-6(9-3-8-5)10-4-11-7/h3-4H,1-2H3,(H,8,9,10,11) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Similars
| Article
PubMed
| >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Sandia National Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant ZAP70 using EEEEYEEEE as substrate by ESI-MS analysis |
J Med Chem 55: 1926-39 (2012)
Article DOI: 10.1021/jm200979x
BindingDB Entry DOI: 10.7270/Q2R212DQ |
More data for this
Ligand-Target Pair | |
Protein-tyrosine kinase 2-beta
(Homo sapiens (Human)) | BDBM50383081

(SCYTONEMIN)Show SMILES Oc1ccc(\C=C2/C3Nc4ccccc4C3=C(C2=O)C2=C3C(=Nc4ccccc34)\C(=C/c3ccc(O)cc3)C2=O)cc1 |c:17,24,t:22| Show InChI InChI=1S/C36H22N2O4/c39-21-13-9-19(10-14-21)17-25-33-29(23-5-1-3-7-27(23)37-33)31(35(25)41)32-30-24-6-2-4-8-28(24)38-34(30)26(36(32)42)18-20-11-15-22(40)16-12-20/h1-18,33,37,39-40H/b25-17+,26-18+ | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Sandia National Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant PYK2 by ESI-MS analysis |
J Med Chem 55: 1926-39 (2012)
Article DOI: 10.1021/jm200979x
BindingDB Entry DOI: 10.7270/Q2R212DQ |
More data for this
Ligand-Target Pair | |
Protein-tyrosine kinase 2-beta
(Homo sapiens (Human)) | BDBM5544
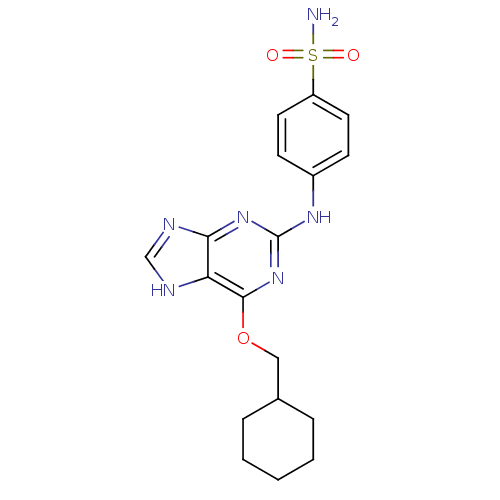
(4-{[6-(cyclohexylmethoxy)-9H-purin-2-yl]amino}benz...)Show SMILES NS(=O)(=O)c1ccc(Nc2nc(OCC3CCCCC3)c3[nH]cnc3n2)cc1 Show InChI InChI=1S/C18H22N6O3S/c19-28(25,26)14-8-6-13(7-9-14)22-18-23-16-15(20-11-21-16)17(24-18)27-10-12-4-2-1-3-5-12/h6-9,11-12H,1-5,10H2,(H2,19,25,26)(H2,20,21,22,23,24) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Sandia National Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant PYK2 by ESI-MS analysis |
J Med Chem 55: 1926-39 (2012)
Article DOI: 10.1021/jm200979x
BindingDB Entry DOI: 10.7270/Q2R212DQ |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase ZAP-70
(Homo sapiens (Human)) | BDBM7680

(4-[2-(5-Bromo-2-oxo-1,2-dihydro-3H-indol-3-ylidene...)Show SMILES NS(=O)(=O)c1ccc(NN=C2C(=O)Nc3ccc(Br)cc23)cc1 |w:9.8| Show InChI InChI=1S/C14H11BrN4O3S/c15-8-1-6-12-11(7-8)13(14(20)17-12)19-18-9-2-4-10(5-3-9)23(16,21)22/h1-7,18H,(H2,16,21,22)(H,17,19,20) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Sandia National Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant ZAP70 using EEEEYEEEE as substrate by ESI-MS analysis |
J Med Chem 55: 1926-39 (2012)
Article DOI: 10.1021/jm200979x
BindingDB Entry DOI: 10.7270/Q2R212DQ |
More data for this
Ligand-Target Pair | |
Protein-tyrosine kinase 2-beta
(Homo sapiens (Human)) | BDBM92422

(CHEMBL407391 | PU09)Show InChI InChI=1S/C7H9N5/c1-12(2)7-5-6(9-3-8-5)10-4-11-7/h3-4H,1-2H3,(H,8,9,10,11) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Similars
| Article
PubMed
| >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Sandia National Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant PYK2 by ESI-MS analysis |
J Med Chem 55: 1926-39 (2012)
Article DOI: 10.1021/jm200979x
BindingDB Entry DOI: 10.7270/Q2R212DQ |
More data for this
Ligand-Target Pair | |
Protein-tyrosine kinase 2-beta
(Homo sapiens (Human)) | BDBM7680

(4-[2-(5-Bromo-2-oxo-1,2-dihydro-3H-indol-3-ylidene...)Show SMILES NS(=O)(=O)c1ccc(NN=C2C(=O)Nc3ccc(Br)cc23)cc1 |w:9.8| Show InChI InChI=1S/C14H11BrN4O3S/c15-8-1-6-12-11(7-8)13(14(20)17-12)19-18-9-2-4-10(5-3-9)23(16,21)22/h1-7,18H,(H2,16,21,22)(H,17,19,20) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Sandia National Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant PYK2 by ESI-MS analysis |
J Med Chem 55: 1926-39 (2012)
Article DOI: 10.1021/jm200979x
BindingDB Entry DOI: 10.7270/Q2R212DQ |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase ZAP-70
(Homo sapiens (Human)) | BDBM4339

((3,4-Dihydroxybenzylidene)malononitrile | 2-[(3,4-...)Show InChI InChI=1S/C10H6N2O2/c11-5-8(6-12)3-7-1-2-9(13)10(14)4-7/h1-4,13-14H | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Sandia National Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant ZAP70 using EEEEYEEEE as substrate by ESI-MS analysis |
J Med Chem 55: 1926-39 (2012)
Article DOI: 10.1021/jm200979x
BindingDB Entry DOI: 10.7270/Q2R212DQ |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase ZAP-70
(Homo sapiens (Human)) | BDBM50341636

((3Z)-N-(3-CHLOROPHENYL)-3-({3,5-DIMETHYL-4-[(4-MET...)Show SMILES CN(c1cccc(Cl)c1)S(=O)(=O)c1ccc2NC(=O)\C(=C/c3[nH]c(C)c(C(=O)N4CCN(C)CC4)c3C)c2c1 Show InChI InChI=1S/C28H30ClN5O4S/c1-17-25(30-18(2)26(17)28(36)34-12-10-32(3)11-13-34)16-23-22-15-21(8-9-24(22)31-27(23)35)39(37,38)33(4)20-7-5-6-19(29)14-20/h5-9,14-16,30H,10-13H2,1-4H3,(H,31,35)/b23-16- | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Sandia National Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant ZAP70 using EEEEYEEEE as substrate by ESI-MS analysis |
J Med Chem 55: 1926-39 (2012)
Article DOI: 10.1021/jm200979x
BindingDB Entry DOI: 10.7270/Q2R212DQ |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase ZAP-70
(Homo sapiens (Human)) | BDBM5544

(4-{[6-(cyclohexylmethoxy)-9H-purin-2-yl]amino}benz...)Show SMILES NS(=O)(=O)c1ccc(Nc2nc(OCC3CCCCC3)c3[nH]cnc3n2)cc1 Show InChI InChI=1S/C18H22N6O3S/c19-28(25,26)14-8-6-13(7-9-14)22-18-23-16-15(20-11-21-16)17(24-18)27-10-12-4-2-1-3-5-12/h6-9,11-12H,1-5,10H2,(H2,19,25,26)(H2,20,21,22,23,24) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Sandia National Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant ZAP70 using EEEEYEEEE as substrate by ESI-MS analysis |
J Med Chem 55: 1926-39 (2012)
Article DOI: 10.1021/jm200979x
BindingDB Entry DOI: 10.7270/Q2R212DQ |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data