 Found 6 hits for monomerid = 92422
Found 6 hits for monomerid = 92422 Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kcal/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
PTK2B protein tyrosine kinase 2 beta (PTK2B)
(Homo sapiens (Human)) | BDBM92422
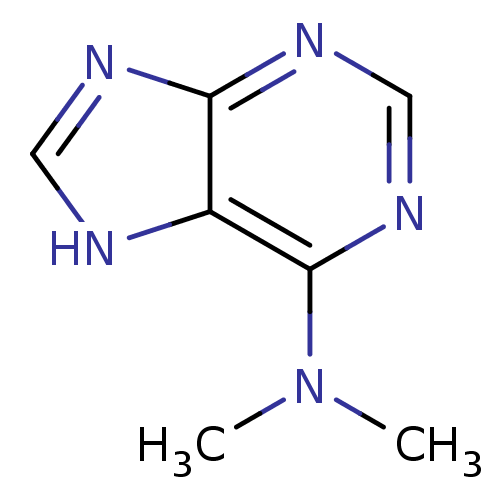
(CHEMBL407391 | PU09)Show InChI InChI=1S/C7H9N5/c1-12(2)7-5-6(9-3-8-5)10-4-11-7/h3-4H,1-2H3,(H,8,9,10,11) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Similars
| Article
PubMed
| >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Sandia National Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant PYK2 by ESI-MS analysis |
J Med Chem 55: 1926-39 (2012)
Article DOI: 10.1021/jm200979x
BindingDB Entry DOI: 10.7270/Q2R212DQ |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase ZAP-70
(Homo sapiens (Human)) | BDBM92422

(CHEMBL407391 | PU09)Show InChI InChI=1S/C7H9N5/c1-12(2)7-5-6(9-3-8-5)10-4-11-7/h3-4H,1-2H3,(H,8,9,10,11) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Similars
| Article
PubMed
| >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Sandia National Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant ZAP70 using EEEEYEEEE as substrate by ESI-MS analysis |
J Med Chem 55: 1926-39 (2012)
Article DOI: 10.1021/jm200979x
BindingDB Entry DOI: 10.7270/Q2R212DQ |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4-kinase type 2-alpha
(Homo sapiens (Human)) | BDBM92422

(CHEMBL407391 | PU09)Show InChI InChI=1S/C7H9N5/c1-12(2)7-5-6(9-3-8-5)10-4-11-7/h3-4H,1-2H3,(H,8,9,10,11) | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Similars
| PubMed
| 1.50E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Smith Kline& French Research Limited
Curated by ChEMBL
| Assay Description
Binding affinity (Ki) against human phosphatidylinositol 4-kinase |
J Med Chem 33: 2073-80 (1990)
BindingDB Entry DOI: 10.7270/Q25T3NQV |
More data for this
Ligand-Target Pair | |
Heat Shock Protein 90 (Hsp90)
(Homo sapiens (Human)) | BDBM92422

(CHEMBL407391 | PU09)Show InChI InChI=1S/C7H9N5/c1-12(2)7-5-6(9-3-8-5)10-4-11-7/h3-4H,1-2H3,(H,8,9,10,11) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
UniProtKB/TrEMBL
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Similars
| PDB
| n/a | n/a | 1.50E+6 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
5-hydroxytryptamine receptor 3A
(Homo sapiens (Human)) | BDBM92422

(CHEMBL407391 | PU09)Show InChI InChI=1S/C7H9N5/c1-12(2)7-5-6(9-3-8-5)10-4-11-7/h3-4H,1-2H3,(H,8,9,10,11) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Similars
| Article
PubMed
| n/a | n/a | >1.00E+5 | n/a | n/a | n/a | n/a | 7.4 | 37 |
University of Copenhagen
| Assay Description
The screening of the compound library at the h5-HT3A-HEK293 cell line and the subsequent functional characterization of the compounds were performed ... |
J Biol Chem 287: 25241-54 (2012)
Article DOI: 10.1074/jbc.M112.360370
BindingDB Entry DOI: 10.7270/Q2N29VHP |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4-kinase type 2-alpha
(Homo sapiens (Human)) | BDBM92422

(CHEMBL407391 | PU09)Show InChI InChI=1S/C7H9N5/c1-12(2)7-5-6(9-3-8-5)10-4-11-7/h3-4H,1-2H3,(H,8,9,10,11) | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Similars
| PubMed
| n/a | n/a | 2.18E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Smith Kline& French Research Limited
Curated by ChEMBL
| Assay Description
Inhibitory activity (IC50) against human phosphatidylinositol 4-kinase at the ATP binding site |
J Med Chem 33: 2073-80 (1990)
BindingDB Entry DOI: 10.7270/Q25T3NQV |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data