 Found 60 hits Enz. Inhib. hit(s) with all data for entry = 50039812
Found 60 hits Enz. Inhib. hit(s) with all data for entry = 50039812 Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Cytochrome P450 3A4
(Homo sapiens (Human)) | BDBM50384612
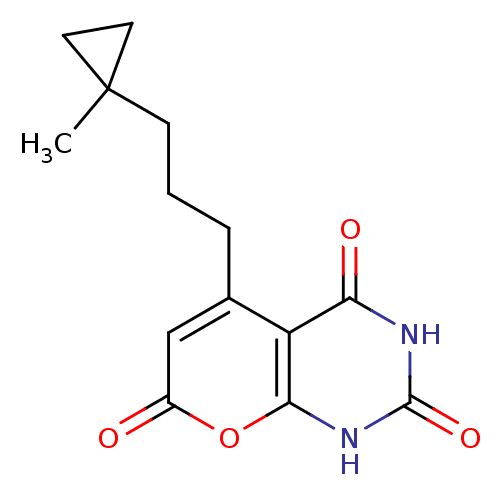
(CHEMBL2036958)Show SMILES CC1(CCCc2cc(=O)oc3[nH]c(=O)[nH]c(=O)c23)CC1 Show InChI InChI=1S/C14H16N2O4/c1-14(5-6-14)4-2-3-8-7-9(17)20-12-10(8)11(18)15-13(19)16-12/h7H,2-6H2,1H3,(H2,15,16,18,19) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | >5.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of human CYP3A4 |
ACS Med Chem Lett 3: 63-68 (2012)
Article DOI: 10.1021/ml200243g
BindingDB Entry DOI: 10.7270/Q29K4C8X |
More data for this
Ligand-Target Pair | |
Cytochrome P450 2C9
(Homo sapiens (Human)) | BDBM50384612

(CHEMBL2036958)Show SMILES CC1(CCCc2cc(=O)oc3[nH]c(=O)[nH]c(=O)c23)CC1 Show InChI InChI=1S/C14H16N2O4/c1-14(5-6-14)4-2-3-8-7-9(17)20-12-10(8)11(18)15-13(19)16-12/h7H,2-6H2,1H3,(H2,15,16,18,19) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | >5.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of human CYP2C9 |
ACS Med Chem Lett 3: 63-68 (2012)
Article DOI: 10.1021/ml200243g
BindingDB Entry DOI: 10.7270/Q29K4C8X |
More data for this
Ligand-Target Pair | |
Cytochrome P450 1A2
(Homo sapiens (Human)) | BDBM50384612

(CHEMBL2036958)Show SMILES CC1(CCCc2cc(=O)oc3[nH]c(=O)[nH]c(=O)c23)CC1 Show InChI InChI=1S/C14H16N2O4/c1-14(5-6-14)4-2-3-8-7-9(17)20-12-10(8)11(18)15-13(19)16-12/h7H,2-6H2,1H3,(H2,15,16,18,19) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | >5.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of human CYP1A2 |
ACS Med Chem Lett 3: 63-68 (2012)
Article DOI: 10.1021/ml200243g
BindingDB Entry DOI: 10.7270/Q29K4C8X |
More data for this
Ligand-Target Pair | |
Cytochrome P450 2D6
(Homo sapiens (Human)) | BDBM50384612

(CHEMBL2036958)Show SMILES CC1(CCCc2cc(=O)oc3[nH]c(=O)[nH]c(=O)c23)CC1 Show InChI InChI=1S/C14H16N2O4/c1-14(5-6-14)4-2-3-8-7-9(17)20-12-10(8)11(18)15-13(19)16-12/h7H,2-6H2,1H3,(H2,15,16,18,19) | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | >5.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of human CYP2D6 |
ACS Med Chem Lett 3: 63-68 (2012)
Article DOI: 10.1021/ml200243g
BindingDB Entry DOI: 10.7270/Q29K4C8X |
More data for this
Ligand-Target Pair | |
Hydroxycarboxylic acid receptor 2
(Homo sapiens (Human)) | BDBM50384617
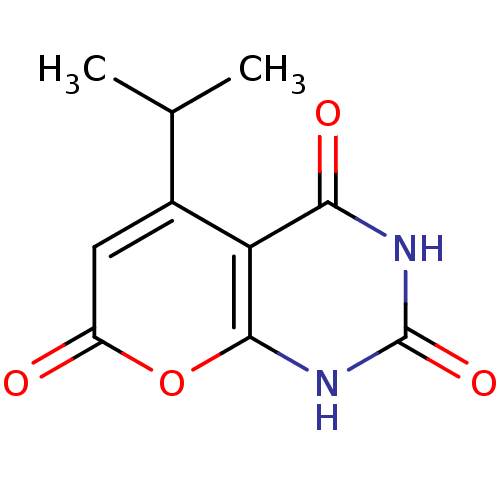
(CHEMBL2036814)Show InChI InChI=1S/C10H10N2O4/c1-4(2)5-3-6(13)16-9-7(5)8(14)11-10(15)12-9/h3-4H,1-2H3,(H2,11,12,14,15) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 220 | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Agonist activity at human recombinant GPR109a expressed in CHO cells assessed as inhibition of forskolin-induced cAMP accumulation |
ACS Med Chem Lett 3: 63-68 (2012)
Article DOI: 10.1021/ml200243g
BindingDB Entry DOI: 10.7270/Q29K4C8X |
More data for this
Ligand-Target Pair | |
Hydroxycarboxylic acid receptor 2
(Homo sapiens (Human)) | BDBM50384618

(CHEMBL2036815)Show InChI InChI=1S/C8H3F3N2O4/c9-8(10,11)2-1-3(14)17-6-4(2)5(15)12-7(16)13-6/h1H,(H2,12,13,15,16) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Agonist activity at human recombinant GPR109a expressed in CHO cells assessed as inhibition of forskolin-induced cAMP accumulation |
ACS Med Chem Lett 3: 63-68 (2012)
Article DOI: 10.1021/ml200243g
BindingDB Entry DOI: 10.7270/Q29K4C8X |
More data for this
Ligand-Target Pair | |
Hydroxycarboxylic acid receptor 2
(Homo sapiens (Human)) | BDBM50384619
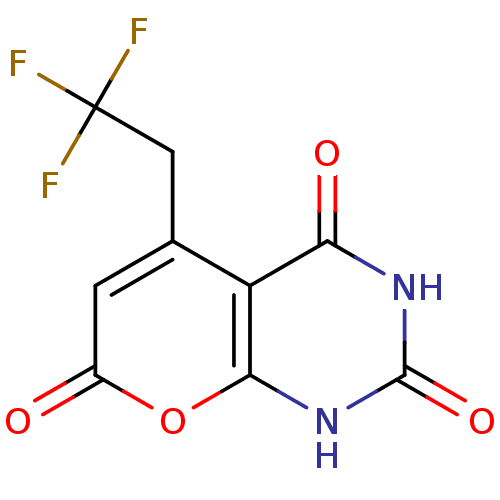
(CHEMBL2036816)Show InChI InChI=1S/C9H5F3N2O4/c10-9(11,12)2-3-1-4(15)18-7-5(3)6(16)13-8(17)14-7/h1H,2H2,(H2,13,14,16,17) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 1.60E+3 | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Agonist activity at human recombinant GPR109a expressed in CHO cells assessed as inhibition of forskolin-induced cAMP accumulation |
ACS Med Chem Lett 3: 63-68 (2012)
Article DOI: 10.1021/ml200243g
BindingDB Entry DOI: 10.7270/Q29K4C8X |
More data for this
Ligand-Target Pair | |
Hydroxycarboxylic acid receptor 2
(Homo sapiens (Human)) | BDBM50384620

(CHEMBL2036817)Show InChI InChI=1S/C13H8N2O4/c16-9-6-8(7-4-2-1-3-5-7)10-11(17)14-13(18)15-12(10)19-9/h1-6H,(H2,14,15,17,18) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Agonist activity at human recombinant GPR109a expressed in CHO cells assessed as inhibition of forskolin-induced cAMP accumulation |
ACS Med Chem Lett 3: 63-68 (2012)
Article DOI: 10.1021/ml200243g
BindingDB Entry DOI: 10.7270/Q29K4C8X |
More data for this
Ligand-Target Pair | |
Hydroxycarboxylic acid receptor 2
(Homo sapiens (Human)) | BDBM50384621
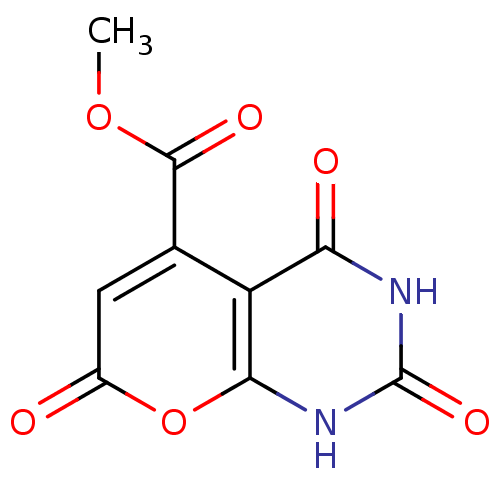
(CHEMBL2036818)Show InChI InChI=1S/C9H6N2O6/c1-16-8(14)3-2-4(12)17-7-5(3)6(13)10-9(15)11-7/h2H,1H3,(H2,10,11,13,15) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Agonist activity at human recombinant GPR109a expressed in CHO cells assessed as inhibition of forskolin-induced cAMP accumulation |
ACS Med Chem Lett 3: 63-68 (2012)
Article DOI: 10.1021/ml200243g
BindingDB Entry DOI: 10.7270/Q29K4C8X |
More data for this
Ligand-Target Pair | |
Hydroxycarboxylic acid receptor 2
(Homo sapiens (Human)) | BDBM50384622

(CHEMBL2036819)Show InChI InChI=1S/C11H10N2O6/c1-2-18-6(14)3-5-4-7(15)19-10-8(5)9(16)12-11(17)13-10/h4H,2-3H2,1H3,(H2,12,13,16,17) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 930 | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Agonist activity at human recombinant GPR109a expressed in CHO cells assessed as inhibition of forskolin-induced cAMP accumulation |
ACS Med Chem Lett 3: 63-68 (2012)
Article DOI: 10.1021/ml200243g
BindingDB Entry DOI: 10.7270/Q29K4C8X |
More data for this
Ligand-Target Pair | |
Hydroxycarboxylic acid receptor 2
(Homo sapiens (Human)) | BDBM50384623
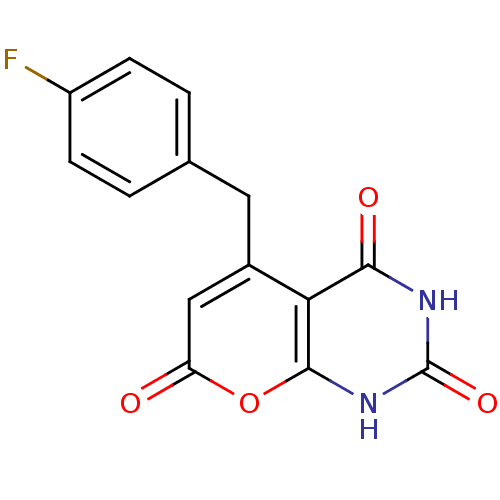
(CHEMBL2036820)Show SMILES Fc1ccc(Cc2cc(=O)oc3[nH]c(=O)[nH]c(=O)c23)cc1 Show InChI InChI=1S/C14H9FN2O4/c15-9-3-1-7(2-4-9)5-8-6-10(18)21-13-11(8)12(19)16-14(20)17-13/h1-4,6H,5H2,(H2,16,17,19,20) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 170 | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Agonist activity at human recombinant GPR109a expressed in CHO cells assessed as inhibition of forskolin-induced cAMP accumulation |
ACS Med Chem Lett 3: 63-68 (2012)
Article DOI: 10.1021/ml200243g
BindingDB Entry DOI: 10.7270/Q29K4C8X |
More data for this
Ligand-Target Pair | |
Hydroxycarboxylic acid receptor 2
(Homo sapiens (Human)) | BDBM50384624
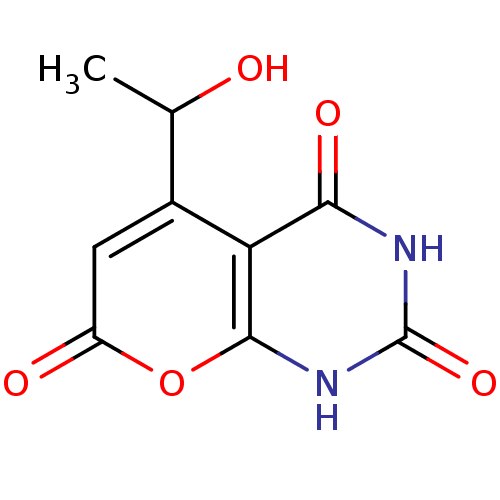
(CHEMBL2036821)Show InChI InChI=1S/C9H8N2O5/c1-3(12)4-2-5(13)16-8-6(4)7(14)10-9(15)11-8/h2-3,12H,1H3,(H2,10,11,14,15) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 1.30E+3 | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Agonist activity at human recombinant GPR109a expressed in CHO cells assessed as inhibition of forskolin-induced cAMP accumulation |
ACS Med Chem Lett 3: 63-68 (2012)
Article DOI: 10.1021/ml200243g
BindingDB Entry DOI: 10.7270/Q29K4C8X |
More data for this
Ligand-Target Pair | |
Hydroxycarboxylic acid receptor 2
(Homo sapiens (Human)) | BDBM50384625
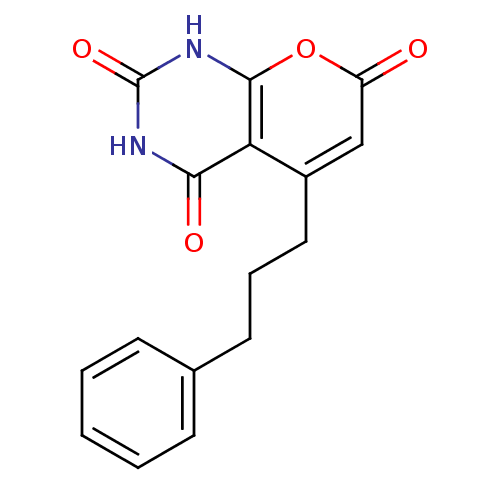
(CHEMBL2036822)Show SMILES O=c1cc(CCCc2ccccc2)c2c([nH]c(=O)[nH]c2=O)o1 Show InChI InChI=1S/C16H14N2O4/c19-12-9-11(8-4-7-10-5-2-1-3-6-10)13-14(20)17-16(21)18-15(13)22-12/h1-3,5-6,9H,4,7-8H2,(H2,17,18,20,21) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 6.10E+3 | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Agonist activity at human recombinant GPR109a expressed in CHO cells assessed as inhibition of forskolin-induced cAMP accumulation |
ACS Med Chem Lett 3: 63-68 (2012)
Article DOI: 10.1021/ml200243g
BindingDB Entry DOI: 10.7270/Q29K4C8X |
More data for this
Ligand-Target Pair | |
Hydroxycarboxylic acid receptor 2
(Homo sapiens (Human)) | BDBM50384626

(CHEMBL2036823)Show InChI InChI=1S/C14H12N2O4S/c17-10-7-8(3-1-4-9-5-2-6-21-9)11-12(18)15-14(19)16-13(11)20-10/h2,5-7H,1,3-4H2,(H2,15,16,18,19) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 26 | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Agonist activity at human recombinant GPR109a expressed in CHO cells assessed as inhibition of forskolin-induced cAMP accumulation |
ACS Med Chem Lett 3: 63-68 (2012)
Article DOI: 10.1021/ml200243g
BindingDB Entry DOI: 10.7270/Q29K4C8X |
More data for this
Ligand-Target Pair | |
Hydroxycarboxylic acid receptor 2
(Homo sapiens (Human)) | BDBM50384627
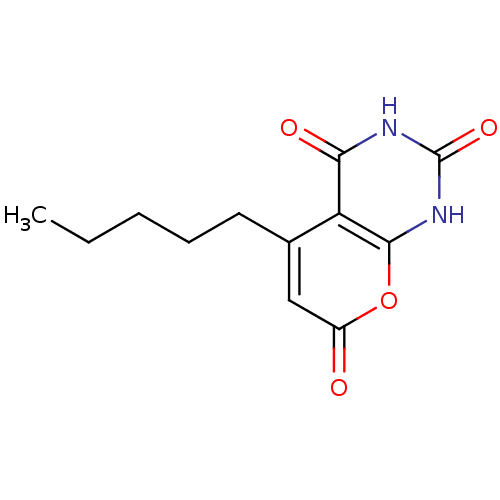
(CHEMBL2036825)Show InChI InChI=1S/C12H14N2O4/c1-2-3-4-5-7-6-8(15)18-11-9(7)10(16)13-12(17)14-11/h6H,2-5H2,1H3,(H2,13,14,16,17) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 19 | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Agonist activity at human recombinant GPR109a expressed in CHO cells assessed as inhibition of forskolin-induced cAMP accumulation |
ACS Med Chem Lett 3: 63-68 (2012)
Article DOI: 10.1021/ml200243g
BindingDB Entry DOI: 10.7270/Q29K4C8X |
More data for this
Ligand-Target Pair | |
Hydroxycarboxylic acid receptor 2
(Homo sapiens (Human)) | BDBM50384628
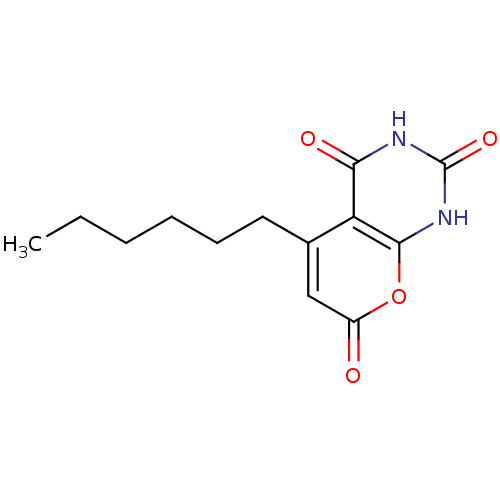
(CHEMBL2036826)Show InChI InChI=1S/C13H16N2O4/c1-2-3-4-5-6-8-7-9(16)19-12-10(8)11(17)14-13(18)15-12/h7H,2-6H2,1H3,(H2,14,15,17,18) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 28 | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Agonist activity at human recombinant GPR109a expressed in CHO cells assessed as inhibition of forskolin-induced cAMP accumulation |
ACS Med Chem Lett 3: 63-68 (2012)
Article DOI: 10.1021/ml200243g
BindingDB Entry DOI: 10.7270/Q29K4C8X |
More data for this
Ligand-Target Pair | |
Hydroxycarboxylic acid receptor 2
(Homo sapiens (Human)) | BDBM50384629

(CHEMBL2036827)Show InChI InChI=1S/C11H12N2O5/c1-17-4-2-3-6-5-7(14)18-10-8(6)9(15)12-11(16)13-10/h5H,2-4H2,1H3,(H2,12,13,15,16) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 110 | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Agonist activity at human recombinant GPR109a expressed in CHO cells assessed as inhibition of forskolin-induced cAMP accumulation |
ACS Med Chem Lett 3: 63-68 (2012)
Article DOI: 10.1021/ml200243g
BindingDB Entry DOI: 10.7270/Q29K4C8X |
More data for this
Ligand-Target Pair | |
Hydroxycarboxylic acid receptor 2
(Homo sapiens (Human)) | BDBM50384630
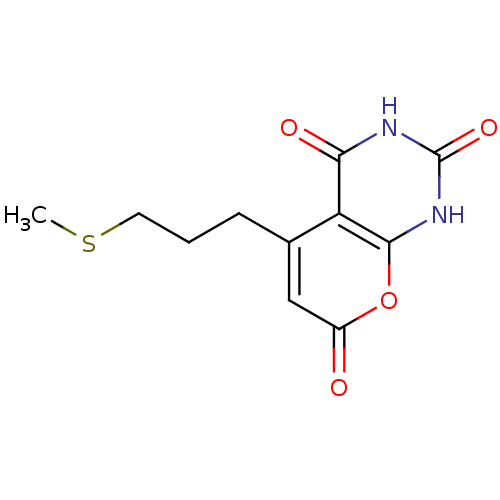
(CHEMBL2036828)Show InChI InChI=1S/C11H12N2O4S/c1-18-4-2-3-6-5-7(14)17-10-8(6)9(15)12-11(16)13-10/h5H,2-4H2,1H3,(H2,12,13,15,16) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 11 | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Agonist activity at human recombinant GPR109a expressed in CHO cells assessed as inhibition of forskolin-induced cAMP accumulation |
ACS Med Chem Lett 3: 63-68 (2012)
Article DOI: 10.1021/ml200243g
BindingDB Entry DOI: 10.7270/Q29K4C8X |
More data for this
Ligand-Target Pair | |
Hydroxycarboxylic acid receptor 2
(Homo sapiens (Human)) | BDBM50384631
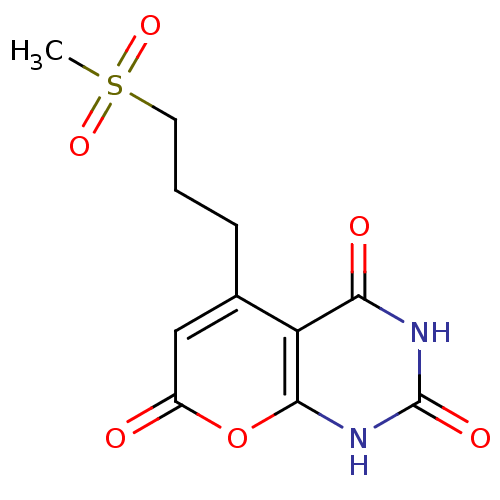
(CHEMBL2036829)Show SMILES CS(=O)(=O)CCCc1cc(=O)oc2[nH]c(=O)[nH]c(=O)c12 Show InChI InChI=1S/C11H12N2O6S/c1-20(17,18)4-2-3-6-5-7(14)19-10-8(6)9(15)12-11(16)13-10/h5H,2-4H2,1H3,(H2,12,13,15,16) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 470 | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Agonist activity at human recombinant GPR109a expressed in CHO cells assessed as inhibition of forskolin-induced cAMP accumulation |
ACS Med Chem Lett 3: 63-68 (2012)
Article DOI: 10.1021/ml200243g
BindingDB Entry DOI: 10.7270/Q29K4C8X |
More data for this
Ligand-Target Pair | |
Hydroxycarboxylic acid receptor 2
(Homo sapiens (Human)) | BDBM50384632

(CHEMBL2036830)Show InChI InChI=1S/C12H14N2O4/c1-3-4-6(2)7-5-8(15)18-11-9(7)10(16)13-12(17)14-11/h5-6H,3-4H2,1-2H3,(H2,13,14,16,17) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 230 | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Agonist activity at human recombinant GPR109a expressed in CHO cells assessed as inhibition of forskolin-induced cAMP accumulation |
ACS Med Chem Lett 3: 63-68 (2012)
Article DOI: 10.1021/ml200243g
BindingDB Entry DOI: 10.7270/Q29K4C8X |
More data for this
Ligand-Target Pair | |
Hydroxycarboxylic acid receptor 2
(Homo sapiens (Human)) | BDBM50384633

(CHEMBL2036947)Show InChI InChI=1S/C12H14N2O4/c1-3-6(2)4-7-5-8(15)18-11-9(7)10(16)13-12(17)14-11/h5-6H,3-4H2,1-2H3,(H2,13,14,16,17) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 310 | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Agonist activity at human recombinant GPR109a expressed in CHO cells assessed as inhibition of forskolin-induced cAMP accumulation |
ACS Med Chem Lett 3: 63-68 (2012)
Article DOI: 10.1021/ml200243g
BindingDB Entry DOI: 10.7270/Q29K4C8X |
More data for this
Ligand-Target Pair | |
Hydroxycarboxylic acid receptor 2
(Homo sapiens (Human)) | BDBM50384634

(CHEMBL2036948)Show InChI InChI=1S/C12H14N2O4/c1-6(2)3-4-7-5-8(15)18-11-9(7)10(16)13-12(17)14-11/h5-6H,3-4H2,1-2H3,(H2,13,14,16,17) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 4 | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Agonist activity at human recombinant GPR109a expressed in CHO cells assessed as inhibition of forskolin-induced cAMP accumulation |
ACS Med Chem Lett 3: 63-68 (2012)
Article DOI: 10.1021/ml200243g
BindingDB Entry DOI: 10.7270/Q29K4C8X |
More data for this
Ligand-Target Pair | |
Hydroxycarboxylic acid receptor 2
(Homo sapiens (Human)) | BDBM50384635
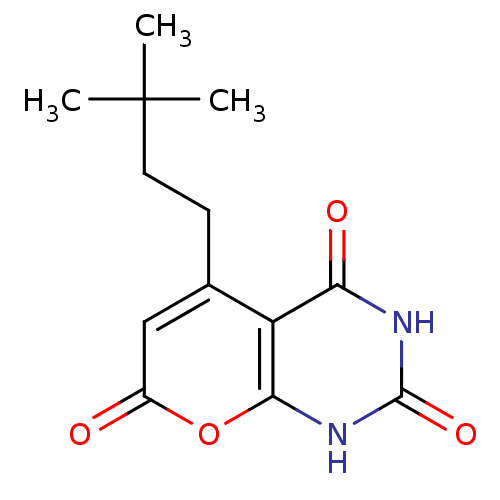
(CHEMBL2036949)Show InChI InChI=1S/C13H16N2O4/c1-13(2,3)5-4-7-6-8(16)19-11-9(7)10(17)14-12(18)15-11/h6H,4-5H2,1-3H3,(H2,14,15,17,18) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Agonist activity at human recombinant GPR109a expressed in CHO cells assessed as inhibition of forskolin-induced cAMP accumulation |
ACS Med Chem Lett 3: 63-68 (2012)
Article DOI: 10.1021/ml200243g
BindingDB Entry DOI: 10.7270/Q29K4C8X |
More data for this
Ligand-Target Pair | |
Hydroxycarboxylic acid receptor 2
(Homo sapiens (Human)) | BDBM50384636

(CHEMBL2036950)Show InChI InChI=1S/C13H16N2O4/c1-7(2)4-3-5-8-6-9(16)19-12-10(8)11(17)14-13(18)15-12/h6-7H,3-5H2,1-2H3,(H2,14,15,17,18) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 5 | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Agonist activity at human recombinant GPR109a expressed in CHO cells assessed as inhibition of forskolin-induced cAMP accumulation |
ACS Med Chem Lett 3: 63-68 (2012)
Article DOI: 10.1021/ml200243g
BindingDB Entry DOI: 10.7270/Q29K4C8X |
More data for this
Ligand-Target Pair | |
Hydroxycarboxylic acid receptor 2
(Homo sapiens (Human)) | BDBM50384637
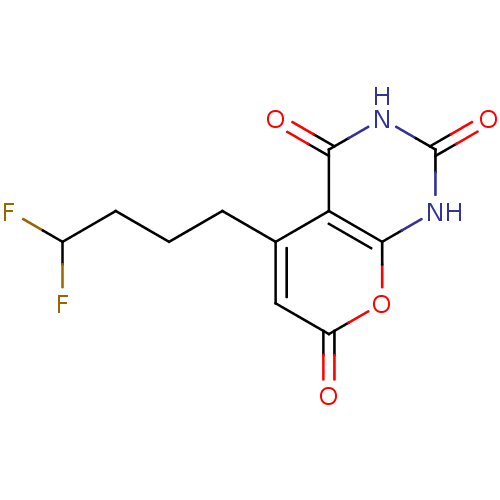
(CHEMBL2036951)Show InChI InChI=1S/C11H10F2N2O4/c12-6(13)3-1-2-5-4-7(16)19-10-8(5)9(17)14-11(18)15-10/h4,6H,1-3H2,(H2,14,15,17,18) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 3 | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Agonist activity at human recombinant GPR109a expressed in CHO cells assessed as inhibition of forskolin-induced cAMP accumulation |
ACS Med Chem Lett 3: 63-68 (2012)
Article DOI: 10.1021/ml200243g
BindingDB Entry DOI: 10.7270/Q29K4C8X |
More data for this
Ligand-Target Pair | |
Hydroxycarboxylic acid receptor 2
(Rattus norvegicus) | BDBM23515

(CHEMBL573 | Niacin | Nicotinic Acid | [5, 6-3H]-ni...)Show InChI InChI=1S/C6H5NO2/c8-6(9)5-2-1-3-7-4-5/h1-4H,(H,8,9) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
Article
PubMed
| n/a | n/a | n/a | n/a | 39 | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Agonist activity at rat GPR109a |
ACS Med Chem Lett 3: 63-68 (2012)
Article DOI: 10.1021/ml200243g
BindingDB Entry DOI: 10.7270/Q29K4C8X |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Hydroxycarboxylic acid receptor 2
(Homo sapiens (Human)) | BDBM50384639
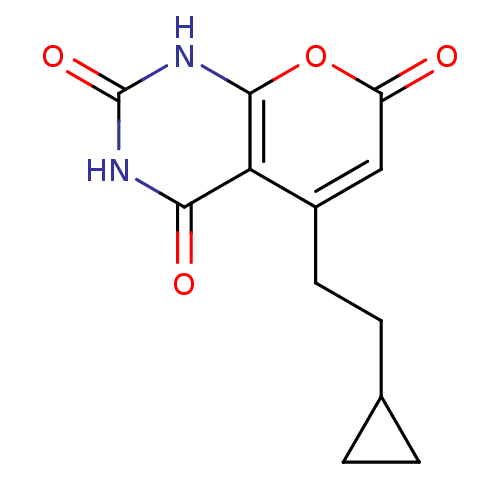
(CHEMBL2036953)Show InChI InChI=1S/C12H12N2O4/c15-8-5-7(4-3-6-1-2-6)9-10(16)13-12(17)14-11(9)18-8/h5-6H,1-4H2,(H2,13,14,16,17) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 13 | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Agonist activity at human recombinant GPR109a expressed in CHO cells assessed as inhibition of forskolin-induced cAMP accumulation |
ACS Med Chem Lett 3: 63-68 (2012)
Article DOI: 10.1021/ml200243g
BindingDB Entry DOI: 10.7270/Q29K4C8X |
More data for this
Ligand-Target Pair | |
Hydroxycarboxylic acid receptor 2
(Homo sapiens (Human)) | BDBM50384640
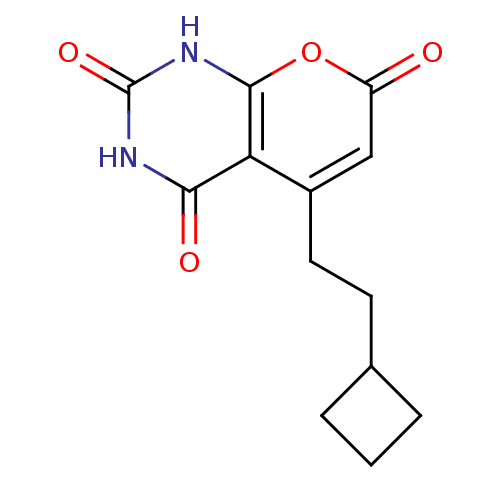
(CHEMBL2036954)Show InChI InChI=1S/C13H14N2O4/c16-9-6-8(5-4-7-2-1-3-7)10-11(17)14-13(18)15-12(10)19-9/h6-7H,1-5H2,(H2,14,15,17,18) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 1 | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Agonist activity at human recombinant GPR109a expressed in CHO cells assessed as inhibition of forskolin-induced cAMP accumulation |
ACS Med Chem Lett 3: 63-68 (2012)
Article DOI: 10.1021/ml200243g
BindingDB Entry DOI: 10.7270/Q29K4C8X |
More data for this
Ligand-Target Pair | |
Hydroxycarboxylic acid receptor 2
(Homo sapiens (Human)) | BDBM50384641
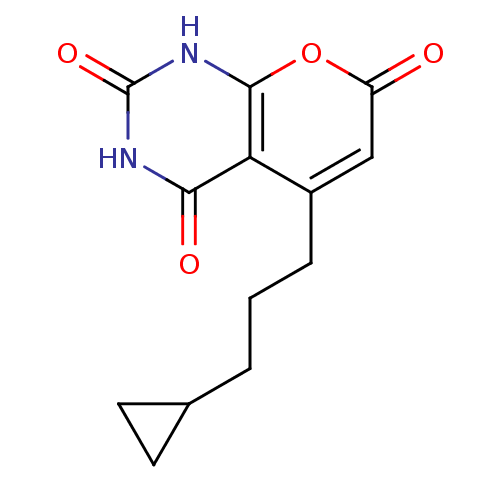
(CHEMBL2036955)Show InChI InChI=1S/C13H14N2O4/c16-9-6-8(3-1-2-7-4-5-7)10-11(17)14-13(18)15-12(10)19-9/h6-7H,1-5H2,(H2,14,15,17,18) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 4 | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Agonist activity at human recombinant GPR109a expressed in CHO cells assessed as inhibition of forskolin-induced cAMP accumulation |
ACS Med Chem Lett 3: 63-68 (2012)
Article DOI: 10.1021/ml200243g
BindingDB Entry DOI: 10.7270/Q29K4C8X |
More data for this
Ligand-Target Pair | |
Hydroxycarboxylic acid receptor 2
(Homo sapiens (Human)) | BDBM50384642
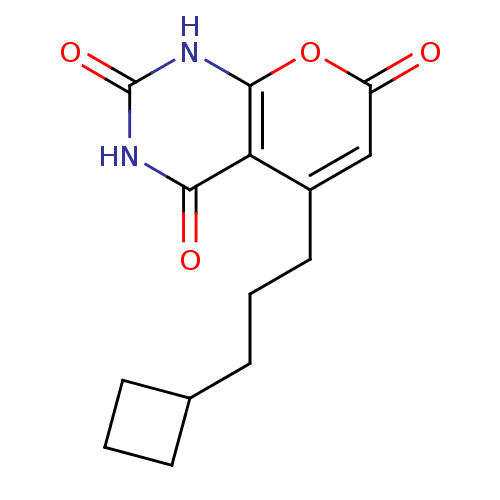
(CHEMBL2036956)Show InChI InChI=1S/C14H16N2O4/c17-10-7-9(6-2-5-8-3-1-4-8)11-12(18)15-14(19)16-13(11)20-10/h7-8H,1-6H2,(H2,15,16,18,19) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 39 | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Agonist activity at human recombinant GPR109a expressed in CHO cells assessed as inhibition of forskolin-induced cAMP accumulation |
ACS Med Chem Lett 3: 63-68 (2012)
Article DOI: 10.1021/ml200243g
BindingDB Entry DOI: 10.7270/Q29K4C8X |
More data for this
Ligand-Target Pair | |
Hydroxycarboxylic acid receptor 2
(Homo sapiens (Human)) | BDBM50384643
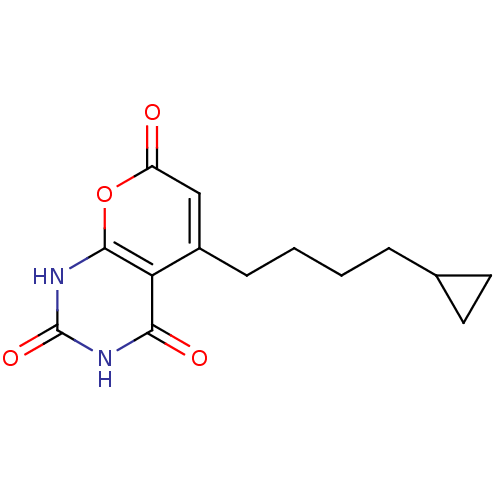
(CHEMBL2036957)Show InChI InChI=1S/C14H16N2O4/c17-10-7-9(4-2-1-3-8-5-6-8)11-12(18)15-14(19)16-13(11)20-10/h7-8H,1-6H2,(H2,15,16,18,19) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 10 | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Agonist activity at human recombinant GPR109a expressed in CHO cells assessed as inhibition of forskolin-induced cAMP accumulation |
ACS Med Chem Lett 3: 63-68 (2012)
Article DOI: 10.1021/ml200243g
BindingDB Entry DOI: 10.7270/Q29K4C8X |
More data for this
Ligand-Target Pair | |
Hydroxycarboxylic acid receptor 2
(Homo sapiens (Human)) | BDBM50384612

(CHEMBL2036958)Show SMILES CC1(CCCc2cc(=O)oc3[nH]c(=O)[nH]c(=O)c23)CC1 Show InChI InChI=1S/C14H16N2O4/c1-14(5-6-14)4-2-3-8-7-9(17)20-12-10(8)11(18)15-13(19)16-12/h7H,2-6H2,1H3,(H2,15,16,18,19) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 2 | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Agonist activity at human recombinant GPR109a expressed in CHO cells assessed as inhibition of forskolin-induced cAMP accumulation |
ACS Med Chem Lett 3: 63-68 (2012)
Article DOI: 10.1021/ml200243g
BindingDB Entry DOI: 10.7270/Q29K4C8X |
More data for this
Ligand-Target Pair | |
Hydroxycarboxylic acid receptor 2
(Homo sapiens (Human)) | BDBM50384644
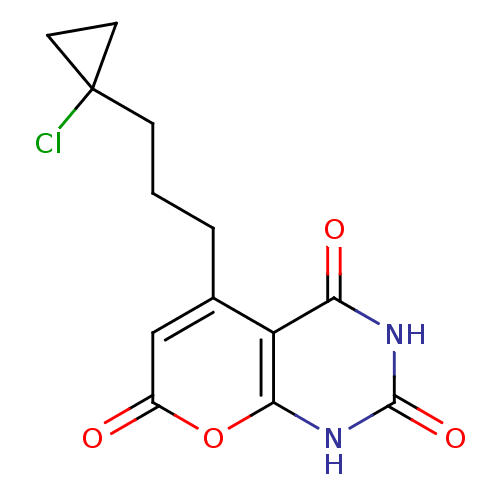
(CHEMBL2036960)Show SMILES ClC1(CCCc2cc(=O)oc3[nH]c(=O)[nH]c(=O)c23)CC1 Show InChI InChI=1S/C13H13ClN2O4/c14-13(4-5-13)3-1-2-7-6-8(17)20-11-9(7)10(18)15-12(19)16-11/h6H,1-5H2,(H2,15,16,18,19) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 7 | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Agonist activity at human recombinant GPR109a expressed in CHO cells assessed as inhibition of forskolin-induced cAMP accumulation |
ACS Med Chem Lett 3: 63-68 (2012)
Article DOI: 10.1021/ml200243g
BindingDB Entry DOI: 10.7270/Q29K4C8X |
More data for this
Ligand-Target Pair | |
Hydroxycarboxylic acid receptor 2
(Homo sapiens (Human)) | BDBM50384645
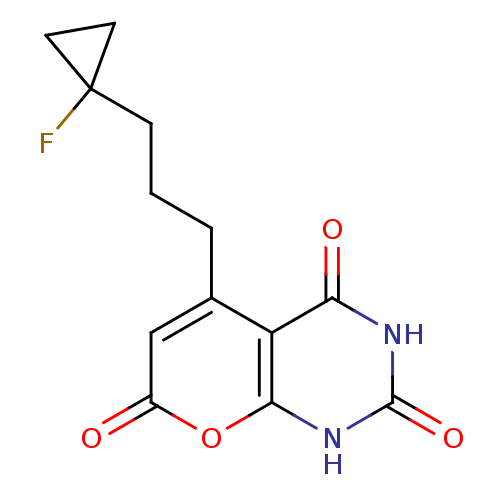
(CHEMBL2035002)Show SMILES FC1(CCCc2cc(=O)oc3[nH]c(=O)[nH]c(=O)c23)CC1 Show InChI InChI=1S/C13H13FN2O4/c14-13(4-5-13)3-1-2-7-6-8(17)20-11-9(7)10(18)15-12(19)16-11/h6H,1-5H2,(H2,15,16,18,19) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 16 | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Agonist activity at human recombinant GPR109a expressed in CHO cells assessed as inhibition of forskolin-induced cAMP accumulation |
ACS Med Chem Lett 3: 63-68 (2012)
Article DOI: 10.1021/ml200243g
BindingDB Entry DOI: 10.7270/Q29K4C8X |
More data for this
Ligand-Target Pair | |
Hydroxycarboxylic acid receptor 2
(Homo sapiens (Human)) | BDBM23515

(CHEMBL573 | Niacin | Nicotinic Acid | [5, 6-3H]-ni...)Show InChI InChI=1S/C6H5NO2/c8-6(9)5-2-1-3-7-4-5/h1-4H,(H,8,9) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| DrugBank
PDB
Article
PubMed
| n/a | n/a | n/a | n/a | 99 | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Agonist activity at human recombinant GPR109a expressed in CHO cells assessed as inhibition of forskolin-induced cAMP accumulation |
ACS Med Chem Lett 3: 63-68 (2012)
Article DOI: 10.1021/ml200243g
BindingDB Entry DOI: 10.7270/Q29K4C8X |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Hydroxycarboxylic acid receptor 3
(Homo sapiens (Human)) | BDBM50384616
(CHEMBL2036813)Show InChI InChI=1S/C11H12N2O4/c1-2-3-4-6-5-7(14)17-10-8(6)9(15)12-11(16)13-10/h5H,2-4H2,1H3,(H2,12,13,15,16) | PDB
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 190 | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Agonist activity at human GPR109b |
ACS Med Chem Lett 3: 63-68 (2012)
Article DOI: 10.1021/ml200243g
BindingDB Entry DOI: 10.7270/Q29K4C8X |
More data for this
Ligand-Target Pair | |
Hydroxycarboxylic acid receptor 3
(Homo sapiens (Human)) | BDBM50384634

(CHEMBL2036948)Show InChI InChI=1S/C12H14N2O4/c1-6(2)3-4-7-5-8(15)18-11-9(7)10(16)13-12(17)14-11/h5-6H,3-4H2,1-2H3,(H2,13,14,16,17) | PDB
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 1.30E+3 | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Agonist activity at human GPR109b |
ACS Med Chem Lett 3: 63-68 (2012)
Article DOI: 10.1021/ml200243g
BindingDB Entry DOI: 10.7270/Q29K4C8X |
More data for this
Ligand-Target Pair | |
Hydroxycarboxylic acid receptor 3
(Homo sapiens (Human)) | BDBM50384637

(CHEMBL2036951)Show InChI InChI=1S/C11H10F2N2O4/c12-6(13)3-1-2-5-4-7(16)19-10-8(5)9(17)14-11(18)15-10/h4,6H,1-3H2,(H2,14,15,17,18) | PDB
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 59 | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Agonist activity at human GPR109b |
ACS Med Chem Lett 3: 63-68 (2012)
Article DOI: 10.1021/ml200243g
BindingDB Entry DOI: 10.7270/Q29K4C8X |
More data for this
Ligand-Target Pair | |
Hydroxycarboxylic acid receptor 3
(Homo sapiens (Human)) | BDBM50384640

(CHEMBL2036954)Show InChI InChI=1S/C13H14N2O4/c16-9-6-8(5-4-7-2-1-3-7)10-11(17)14-13(18)15-12(10)19-9/h6-7H,1-5H2,(H2,14,15,17,18) | PDB
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 67 | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Agonist activity at human GPR109b |
ACS Med Chem Lett 3: 63-68 (2012)
Article DOI: 10.1021/ml200243g
BindingDB Entry DOI: 10.7270/Q29K4C8X |
More data for this
Ligand-Target Pair | |
Hydroxycarboxylic acid receptor 3
(Homo sapiens (Human)) | BDBM50384641

(CHEMBL2036955)Show InChI InChI=1S/C13H14N2O4/c16-9-6-8(3-1-2-7-4-5-7)10-11(17)14-13(18)15-12(10)19-9/h6-7H,1-5H2,(H2,14,15,17,18) | PDB
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 140 | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Agonist activity at human GPR109b |
ACS Med Chem Lett 3: 63-68 (2012)
Article DOI: 10.1021/ml200243g
BindingDB Entry DOI: 10.7270/Q29K4C8X |
More data for this
Ligand-Target Pair | |
Hydroxycarboxylic acid receptor 3
(Homo sapiens (Human)) | BDBM50384612

(CHEMBL2036958)Show SMILES CC1(CCCc2cc(=O)oc3[nH]c(=O)[nH]c(=O)c23)CC1 Show InChI InChI=1S/C14H16N2O4/c1-14(5-6-14)4-2-3-8-7-9(17)20-12-10(8)11(18)15-13(19)16-12/h7H,2-6H2,1H3,(H2,15,16,18,19) | PDB
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 96 | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Agonist activity at human GPR109b |
ACS Med Chem Lett 3: 63-68 (2012)
Article DOI: 10.1021/ml200243g
BindingDB Entry DOI: 10.7270/Q29K4C8X |
More data for this
Ligand-Target Pair | |
Hydroxycarboxylic acid receptor 3
(Homo sapiens (Human)) | BDBM23515

(CHEMBL573 | Niacin | Nicotinic Acid | [5, 6-3H]-ni...)Show InChI InChI=1S/C6H5NO2/c8-6(9)5-2-1-3-7-4-5/h1-4H,(H,8,9) | PDB
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| DrugBank
PDB
Article
PubMed
| n/a | n/a | n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Agonist activity at human GPR109b |
ACS Med Chem Lett 3: 63-68 (2012)
Article DOI: 10.1021/ml200243g
BindingDB Entry DOI: 10.7270/Q29K4C8X |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Hydroxycarboxylic acid receptor 2
(Rattus norvegicus) | BDBM50384616

(CHEMBL2036813)Show InChI InChI=1S/C11H12N2O4/c1-2-3-4-6-5-7(14)17-10-8(6)9(15)12-11(16)13-10/h5H,2-4H2,1H3,(H2,12,13,15,16) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 8 | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Agonist activity at rat GPR109a |
ACS Med Chem Lett 3: 63-68 (2012)
Article DOI: 10.1021/ml200243g
BindingDB Entry DOI: 10.7270/Q29K4C8X |
More data for this
Ligand-Target Pair | |
Hydroxycarboxylic acid receptor 2
(Rattus norvegicus) | BDBM50384634

(CHEMBL2036948)Show InChI InChI=1S/C12H14N2O4/c1-6(2)3-4-7-5-8(15)18-11-9(7)10(16)13-12(17)14-11/h5-6H,3-4H2,1-2H3,(H2,13,14,16,17) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 4 | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Agonist activity at rat GPR109a |
ACS Med Chem Lett 3: 63-68 (2012)
Article DOI: 10.1021/ml200243g
BindingDB Entry DOI: 10.7270/Q29K4C8X |
More data for this
Ligand-Target Pair | |
Hydroxycarboxylic acid receptor 2
(Rattus norvegicus) | BDBM50384637

(CHEMBL2036951)Show InChI InChI=1S/C11H10F2N2O4/c12-6(13)3-1-2-5-4-7(16)19-10-8(5)9(17)14-11(18)15-10/h4,6H,1-3H2,(H2,14,15,17,18) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 2 | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Agonist activity at rat GPR109a |
ACS Med Chem Lett 3: 63-68 (2012)
Article DOI: 10.1021/ml200243g
BindingDB Entry DOI: 10.7270/Q29K4C8X |
More data for this
Ligand-Target Pair | |
Hydroxycarboxylic acid receptor 2
(Rattus norvegicus) | BDBM50384640

(CHEMBL2036954)Show InChI InChI=1S/C13H14N2O4/c16-9-6-8(5-4-7-2-1-3-7)10-11(17)14-13(18)15-12(10)19-9/h6-7H,1-5H2,(H2,14,15,17,18) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 5 | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Agonist activity at rat GPR109a |
ACS Med Chem Lett 3: 63-68 (2012)
Article DOI: 10.1021/ml200243g
BindingDB Entry DOI: 10.7270/Q29K4C8X |
More data for this
Ligand-Target Pair | |
Hydroxycarboxylic acid receptor 2
(Rattus norvegicus) | BDBM50384641

(CHEMBL2036955)Show InChI InChI=1S/C13H14N2O4/c16-9-6-8(3-1-2-7-4-5-7)10-11(17)14-13(18)15-12(10)19-9/h6-7H,1-5H2,(H2,14,15,17,18) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 2 | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Agonist activity at rat GPR109a |
ACS Med Chem Lett 3: 63-68 (2012)
Article DOI: 10.1021/ml200243g
BindingDB Entry DOI: 10.7270/Q29K4C8X |
More data for this
Ligand-Target Pair | |
Hydroxycarboxylic acid receptor 2
(Rattus norvegicus) | BDBM50384612

(CHEMBL2036958)Show SMILES CC1(CCCc2cc(=O)oc3[nH]c(=O)[nH]c(=O)c23)CC1 Show InChI InChI=1S/C14H16N2O4/c1-14(5-6-14)4-2-3-8-7-9(17)20-12-10(8)11(18)15-13(19)16-12/h7H,2-6H2,1H3,(H2,15,16,18,19) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 8 | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Agonist activity at rat GPR109a |
ACS Med Chem Lett 3: 63-68 (2012)
Article DOI: 10.1021/ml200243g
BindingDB Entry DOI: 10.7270/Q29K4C8X |
More data for this
Ligand-Target Pair | |
Hydroxycarboxylic acid receptor 2
(Mus musculus) | BDBM50384616

(CHEMBL2036813)Show InChI InChI=1S/C11H12N2O4/c1-2-3-4-6-5-7(14)17-10-8(6)9(15)12-11(16)13-10/h5H,2-4H2,1H3,(H2,12,13,15,16) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 12 | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Agonist activity at mouse GPR109a |
ACS Med Chem Lett 3: 63-68 (2012)
Article DOI: 10.1021/ml200243g
BindingDB Entry DOI: 10.7270/Q29K4C8X |
More data for this
Ligand-Target Pair | |
Hydroxycarboxylic acid receptor 2
(Mus musculus) | BDBM50384640

(CHEMBL2036954)Show InChI InChI=1S/C13H14N2O4/c16-9-6-8(5-4-7-2-1-3-7)10-11(17)14-13(18)15-12(10)19-9/h6-7H,1-5H2,(H2,14,15,17,18) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 4 | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Agonist activity at mouse GPR109a |
ACS Med Chem Lett 3: 63-68 (2012)
Article DOI: 10.1021/ml200243g
BindingDB Entry DOI: 10.7270/Q29K4C8X |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data