 Found 83 hits Enz. Inhib. hit(s) with all data for entry = 50018161
Found 83 hits Enz. Inhib. hit(s) with all data for entry = 50018161 Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit alpha isoform
(Homo sapiens (Human)) | BDBM50240975
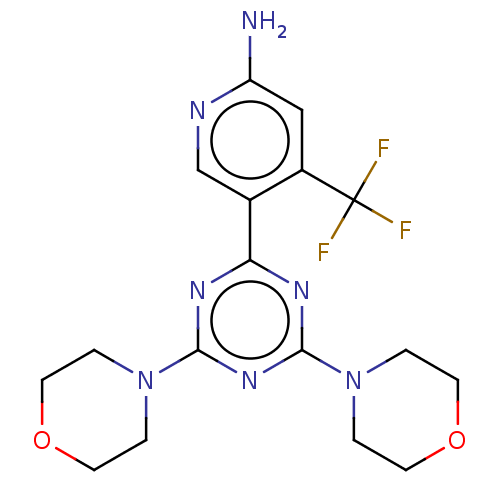
(CHEMBL4084907)Show SMILES Nc1cc(c(cn1)-c1nc(nc(n1)N1CCOCC1)N1CCOCC1)C(F)(F)F Show InChI InChI=1S/C17H20F3N7O2/c18-17(19,20)12-9-13(21)22-10-11(12)14-23-15(26-1-5-28-6-2-26)25-16(24-14)27-3-7-29-8-4-27/h9-10H,1-8H2,(H2,21,22) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
PDB
UniChem
Similars
| | 17 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Antagonist activity was determined against beta-1 adrenergic receptor in spontaneously beating rat atria |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase mTOR
(Homo sapiens (Human)) | BDBM50240975

(CHEMBL4084907)Show SMILES Nc1cc(c(cn1)-c1nc(nc(n1)N1CCOCC1)N1CCOCC1)C(F)(F)F Show InChI InChI=1S/C17H20F3N7O2/c18-17(19,20)12-9-13(21)22-10-11(12)14-23-15(26-1-5-28-6-2-26)25-16(24-14)27-3-7-29-8-4-27/h9-10H,1-8H2,(H2,21,22) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
PDB
UniChem
Similars
| | 62 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Antagonist activity was determined against beta-1 adrenergic receptor in spontaneously beating rat atria |
Citation and Details
|
More data for this
Ligand-Target Pair | |
RAC-alpha serine/threonine-protein kinase
(Homo sapiens (Human)) | BDBM50170284
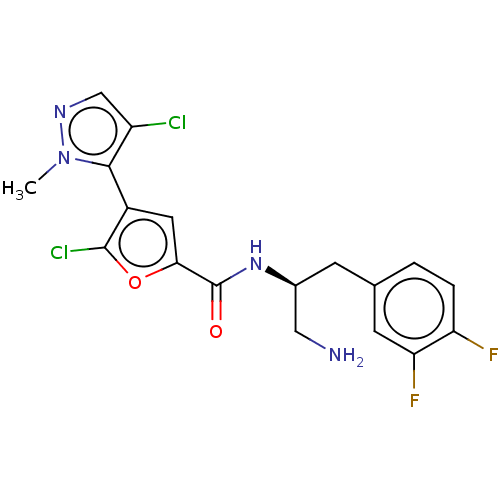
(GSK2141795 | GSK2141795C | Uprosertib)Show SMILES Cn1ncc(Cl)c1-c1cc(oc1Cl)C(=O)N[C@H](CN)Cc1ccc(F)c(F)c1 |r,wD:16.18,(2.37,-.54,;3.52,.49,;3.36,2.02,;4.77,2.65,;5.8,1.51,;7.33,1.67,;5.03,.17,;5.65,-1.23,;7.16,-1.55,;7.32,-3.09,;5.91,-3.71,;4.88,-2.57,;3.35,-2.73,;8.65,-3.86,;9.99,-3.09,;8.65,-5.4,;9.99,-6.17,;11.32,-5.4,;12.65,-6.17,;9.99,-7.71,;11.32,-8.48,;12.65,-7.71,;13.99,-8.48,;13.99,-10.02,;15.32,-10.79,;12.65,-10.79,;12.65,-12.33,;11.32,-10.02,)| Show InChI InChI=1S/C18H16Cl2F2N4O2/c1-26-16(12(19)8-24-26)11-6-15(28-17(11)20)18(27)25-10(7-23)4-9-2-3-13(21)14(22)5-9/h2-3,5-6,8,10H,4,7,23H2,1H3,(H,25,27)/t10-/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
Similars
| | n/a | n/a | 0.0660 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of choline acetyltransferase (ChAT) activity |
Citation and Details
|
More data for this
Ligand-Target Pair | |
RAC-alpha serine/threonine-protein kinase
(Homo sapiens (Human)) | BDBM50502477
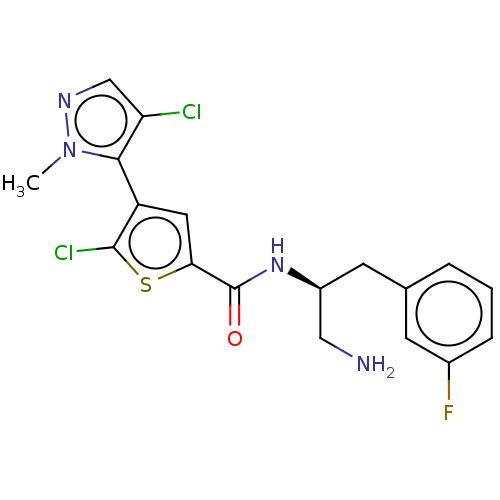
(ASB-183 | ASB183 | Afuresertib | GSK-2110183C | GS...)Show SMILES [H][C@@](CN)(Cc1cccc(F)c1)NC(=O)c1cc(c(Cl)s1)-c1c(Cl)cnn1C |r,wU:1.1,wD:1.0,(11.52,-5.94,;10.83,-4.68,;10.06,-6.01,;10.83,-7.35,;12.17,-3.88,;13.49,-4.64,;13.48,-6.2,;14.82,-6.95,;16.15,-6.19,;16.19,-4.64,;17.46,-3.84,;14.79,-3.91,;9.41,-3.84,;8.08,-4.6,;8.11,-6.13,;6.85,-3.92,;5.41,-4.51,;4.4,-3.4,;5.15,-2.04,;4.33,-.64,;6.62,-2.35,;2.85,-3.54,;1.85,-2.41,;2.36,-.88,;.42,-3.01,;.5,-4.52,;2.04,-4.83,;2.44,-6.32,)| Show InChI InChI=1S/C18H17Cl2FN4OS/c1-25-16(14(19)9-23-25)13-7-15(27-17(13)20)18(26)24-12(8-22)6-10-3-2-4-11(21)5-10/h2-5,7,9,12H,6,8,22H2,1H3,(H,24,26)/t12-/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
| | n/a | n/a | 0.0800 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of acetylcholinesterase (AChE) activity |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase mTOR
(Homo sapiens (Human)) | BDBM50348452
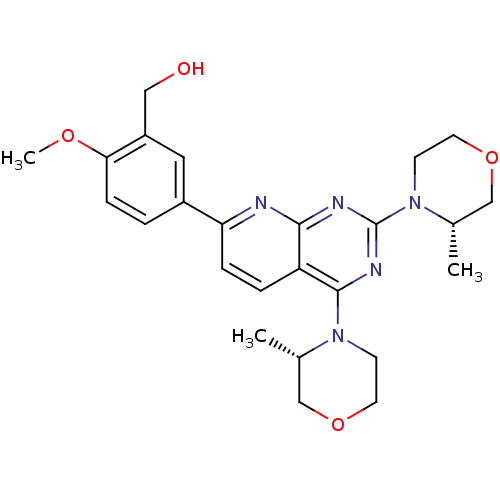
(AZD-8055 | CHEMBL1801204 | US9102670, 1a)Show SMILES COc1ccc(cc1CO)-c1ccc2c(nc(nc2n1)N1CCOC[C@@H]1C)N1CCOC[C@@H]1C |r| Show InChI InChI=1S/C25H31N5O4/c1-16-14-33-10-8-29(16)24-20-5-6-21(18-4-7-22(32-3)19(12-18)13-31)26-23(20)27-25(28-24)30-9-11-34-15-17(30)2/h4-7,12,16-17,31H,8-11,13-15H2,1-3H3/t16-,17-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| | n/a | n/a | 0.130 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Antagonist activity was determined against beta-1 adrenergic receptor in spontaneously beating rat atria |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase mTOR
(Homo sapiens (Human)) | BDBM50405629
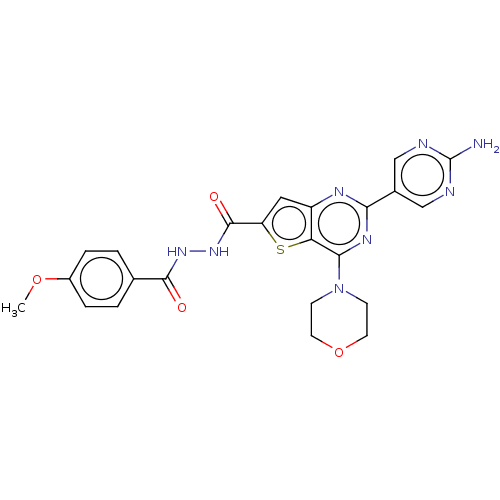
(CHEMBL5278435)Show InChI InChI=1S/C14H21NO2/c16-14(13-8-4-5-9-15-13)11-17-10-12-6-2-1-3-7-12/h1-3,6-7,13-16H,4-5,8-11H2/t13-,14+/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| | n/a | n/a | 0.460 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Antagonist activity was determined against beta-1 adrenergic receptor in spontaneously beating rat atria |
Citation and Details
|
More data for this
Ligand-Target Pair | |
RAC-alpha serine/threonine-protein kinase
(Homo sapiens (Human)) | BDBM50405634
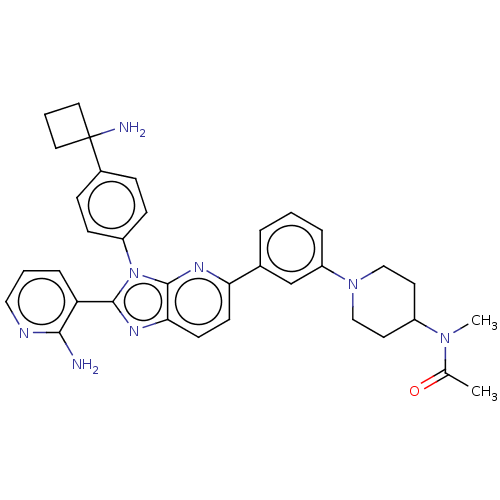
(VEVORISERTIB | Vevorisertib)Show InChI InChI=1S/C13H21NO2/c1-11(2)14(3)9-12(15)10-16-13-7-5-4-6-8-13/h4-8,11-12,15H,9-10H2,1-3H3 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| | n/a | n/a | 0.550 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of choline acetyltransferase (ChAT) activity |
Citation and Details
|
More data for this
Ligand-Target Pair | |
RAC-beta serine/threonine-protein kinase
(Homo sapiens (Human)) | BDBM50405634

(VEVORISERTIB | Vevorisertib)Show InChI InChI=1S/C13H21NO2/c1-11(2)14(3)9-12(15)10-16-13-7-5-4-6-8-13/h4-8,11-12,15H,9-10H2,1-3H3 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| | n/a | n/a | 0.810 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of acetylcholinesterase (AChE) activity |
Citation and Details
|
More data for this
Ligand-Target Pair | |
RAC-alpha serine/threonine-protein kinase
(Homo sapiens (Human)) | BDBM431867
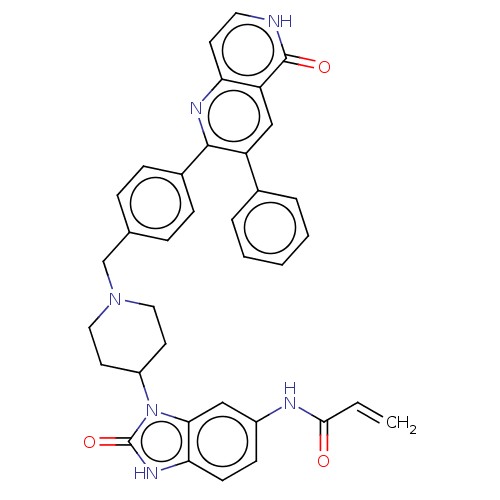
(US10550114, Compound 1a)Show SMILES C=CC(=O)Nc1ccc2[nH]c(=O)n(C3CCN(Cc4ccc(cc4)-c4nc5cc[nH]c(=O)c5cc4-c4ccccc4)CC3)c2c1 Show InChI InChI=1S/C36H32N6O3/c1-2-33(43)38-26-12-13-31-32(20-26)42(36(45)40-31)27-15-18-41(19-16-27)22-23-8-10-25(11-9-23)34-28(24-6-4-3-5-7-24)21-29-30(39-34)14-17-37-35(29)44/h2-14,17,20-21,27H,1,15-16,18-19,22H2,(H,37,44)(H,38,43)(H,40,45) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
| | n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of acetylcholinesterase (AChE) activity |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit delta isoform
(Homo sapiens (Human)) | BDBM50405628
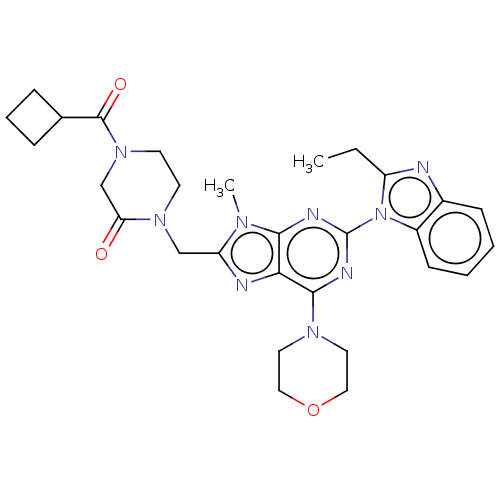
(CHEMBL5272165)Show InChI InChI=1S/C17H21NO2/c19-17(16-7-3-4-10-18-16)12-20-15-9-8-13-5-1-2-6-14(13)11-15/h1-2,5-6,8-9,11,16-19H,3-4,7,10,12H2/t16-,17+/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| | n/a | n/a | 1.20 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Cardioselectivity for the beta-2 adrenergic receptor was determined against isoprenaline (antagonism) in isolated rat atria |
Citation and Details
|
More data for this
Ligand-Target Pair | |
RAC-gamma serine/threonine-protein kinase
(Homo sapiens (Human)) | BDBM50405634

(VEVORISERTIB | Vevorisertib)Show InChI InChI=1S/C13H21NO2/c1-11(2)14(3)9-12(15)10-16-13-7-5-4-6-8-13/h4-8,11-12,15H,9-10H2,1-3H3 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| | n/a | n/a | 1.30 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of choline acetyltransferase (ChAT) activity |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Histone deacetylase 1
(Homo sapiens (Human)) | BDBM50503943
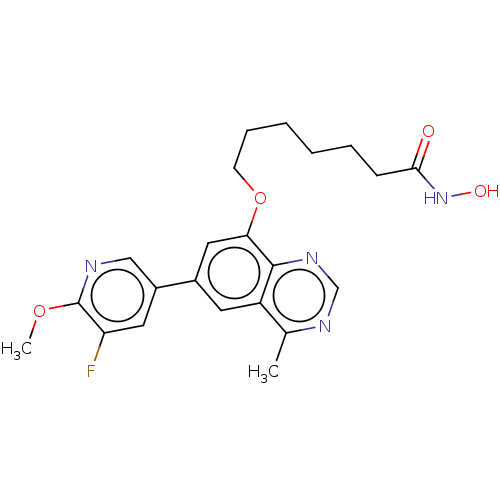
(CHEMBL4536105)Show SMILES COc1ncc(cc1F)-c1cc(OCCCCCCC(=O)NO)c2ncnc(C)c2c1 Show InChI InChI=1S/C22H25FN4O4/c1-14-17-9-15(16-10-18(23)22(30-2)24-12-16)11-19(21(17)26-13-25-14)31-8-6-4-3-5-7-20(28)27-29/h9-13,29H,3-8H2,1-2H3,(H,27,28) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
| | n/a | n/a | 1.40 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Antagonist activity was determined against beta-1 adrenergic receptor in spontaneously beating rat atria |
Citation and Details
|
More data for this
Ligand-Target Pair | |
RAC-beta serine/threonine-protein kinase
(Homo sapiens (Human)) | BDBM50170284

(GSK2141795 | GSK2141795C | Uprosertib)Show SMILES Cn1ncc(Cl)c1-c1cc(oc1Cl)C(=O)N[C@H](CN)Cc1ccc(F)c(F)c1 |r,wD:16.18,(2.37,-.54,;3.52,.49,;3.36,2.02,;4.77,2.65,;5.8,1.51,;7.33,1.67,;5.03,.17,;5.65,-1.23,;7.16,-1.55,;7.32,-3.09,;5.91,-3.71,;4.88,-2.57,;3.35,-2.73,;8.65,-3.86,;9.99,-3.09,;8.65,-5.4,;9.99,-6.17,;11.32,-5.4,;12.65,-6.17,;9.99,-7.71,;11.32,-8.48,;12.65,-7.71,;13.99,-8.48,;13.99,-10.02,;15.32,-10.79,;12.65,-10.79,;12.65,-12.33,;11.32,-10.02,)| Show InChI InChI=1S/C18H16Cl2F2N4O2/c1-26-16(12(19)8-24-26)11-6-15(28-17(11)20)18(27)25-10(7-23)4-9-2-3-13(21)14(22)5-9/h2-3,5-6,8,10H,4,7,23H2,1H3,(H,25,27)/t10-/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
Similars
| | n/a | n/a | 1.40 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of choline acetyltransferase (ChAT) activity |
Citation and Details
|
More data for this
Ligand-Target Pair | |
RAC-gamma serine/threonine-protein kinase
(Homo sapiens (Human)) | BDBM50170284

(GSK2141795 | GSK2141795C | Uprosertib)Show SMILES Cn1ncc(Cl)c1-c1cc(oc1Cl)C(=O)N[C@H](CN)Cc1ccc(F)c(F)c1 |r,wD:16.18,(2.37,-.54,;3.52,.49,;3.36,2.02,;4.77,2.65,;5.8,1.51,;7.33,1.67,;5.03,.17,;5.65,-1.23,;7.16,-1.55,;7.32,-3.09,;5.91,-3.71,;4.88,-2.57,;3.35,-2.73,;8.65,-3.86,;9.99,-3.09,;8.65,-5.4,;9.99,-6.17,;11.32,-5.4,;12.65,-6.17,;9.99,-7.71,;11.32,-8.48,;12.65,-7.71,;13.99,-8.48,;13.99,-10.02,;15.32,-10.79,;12.65,-10.79,;12.65,-12.33,;11.32,-10.02,)| Show InChI InChI=1S/C18H16Cl2F2N4O2/c1-26-16(12(19)8-24-26)11-6-15(28-17(11)20)18(27)25-10(7-23)4-9-2-3-13(21)14(22)5-9/h2-3,5-6,8,10H,4,7,23H2,1H3,(H,25,27)/t10-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
Similars
| | n/a | n/a | 1.5 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of choline acetyltransferase (ChAT) activity |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit delta isoform
(Mus musculus (Mouse)) | BDBM50380313
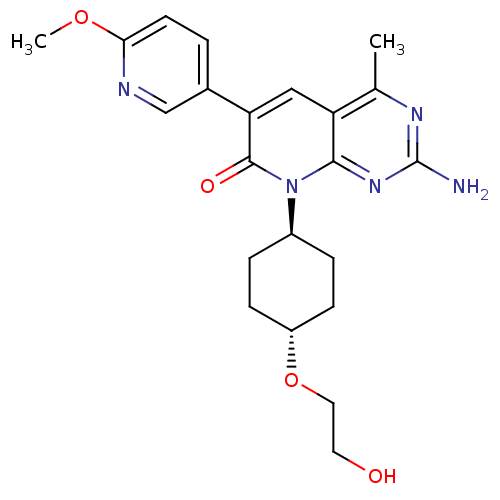
(CHEMBL1234354 | US8633204, 286)Show SMILES COc1ccc(cn1)-c1cc2c(C)nc(N)nc2n([C@H]2CC[C@@H](CC2)OCCO)c1=O |r,wU:19.20,wD:22.27,(7.3,4.56,;5.97,5.33,;4.64,4.56,;3.3,5.33,;1.97,4.56,;1.97,3.02,;3.3,2.25,;4.64,3.02,;.64,2.25,;-.7,3.02,;-2.03,2.25,;-3.37,3.02,;-3.37,4.56,;-4.7,2.25,;-4.7,.71,;-6.03,-.06,;-3.37,-.06,;-2.03,.71,;-.7,-.06,;-.7,-1.6,;.64,-2.37,;.64,-3.91,;-.7,-4.68,;-2.03,-3.91,;-2.03,-2.37,;-.7,-6.22,;.64,-6.99,;.64,-8.53,;1.97,-9.3,;.64,.71,;1.97,-.06,)| Show InChI InChI=1S/C22H27N5O4/c1-13-17-11-18(14-3-8-19(30-2)24-12-14)21(29)27(20(17)26-22(23)25-13)15-4-6-16(7-5-15)31-10-9-28/h3,8,11-12,15-16,28H,4-7,9-10H2,1-2H3,(H2,23,25,26)/t15-,16- | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Similars
| | n/a | n/a | 1.60 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Antagonist activity was determined against beta-1 adrenergic receptor in spontaneously beating rat atria |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit delta isoform
(Homo sapiens (Human)) | BDBM50380313

(CHEMBL1234354 | US8633204, 286)Show SMILES COc1ccc(cn1)-c1cc2c(C)nc(N)nc2n([C@H]2CC[C@@H](CC2)OCCO)c1=O |r,wU:19.20,wD:22.27,(7.3,4.56,;5.97,5.33,;4.64,4.56,;3.3,5.33,;1.97,4.56,;1.97,3.02,;3.3,2.25,;4.64,3.02,;.64,2.25,;-.7,3.02,;-2.03,2.25,;-3.37,3.02,;-3.37,4.56,;-4.7,2.25,;-4.7,.71,;-6.03,-.06,;-3.37,-.06,;-2.03,.71,;-.7,-.06,;-.7,-1.6,;.64,-2.37,;.64,-3.91,;-.7,-4.68,;-2.03,-3.91,;-2.03,-2.37,;-.7,-6.22,;.64,-6.99,;.64,-8.53,;1.97,-9.3,;.64,.71,;1.97,-.06,)| Show InChI InChI=1S/C22H27N5O4/c1-13-17-11-18(14-3-8-19(30-2)24-12-14)21(29)27(20(17)26-22(23)25-13)15-4-6-16(7-5-15)31-10-9-28/h3,8,11-12,15-16,28H,4-7,9-10H2,1-2H3,(H2,23,25,26)/t15-,16- | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Similars
| | n/a | n/a | 1.60 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of choline acetyltransferase (ChAT) activity |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Histone deacetylase 1
(Homo sapiens (Human)) | BDBM50405632
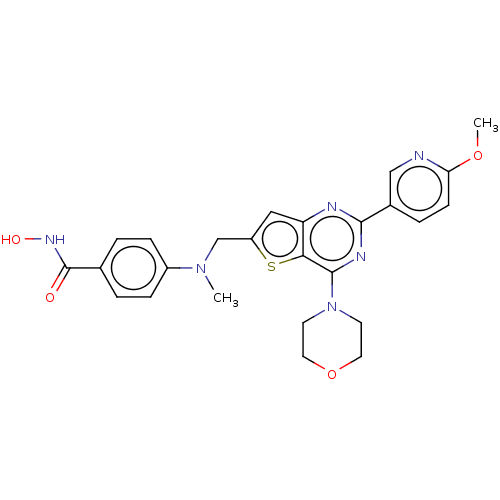
(CHEMBL5278968)Show InChI InChI=1S/C16H21NO2/c1-12(2)17-10-15(18)11-19-16-8-7-13-5-3-4-6-14(13)9-16/h3-9,12,15,17-18H,10-11H2,1-2H3 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Similars
| | n/a | n/a | 1.70 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Antagonist activity was determined against beta-1 adrenergic receptor in spontaneously beating rat atria |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit alpha isoform
(Mus musculus (Mouse)) | BDBM50380313

(CHEMBL1234354 | US8633204, 286)Show SMILES COc1ccc(cn1)-c1cc2c(C)nc(N)nc2n([C@H]2CC[C@@H](CC2)OCCO)c1=O |r,wU:19.20,wD:22.27,(7.3,4.56,;5.97,5.33,;4.64,4.56,;3.3,5.33,;1.97,4.56,;1.97,3.02,;3.3,2.25,;4.64,3.02,;.64,2.25,;-.7,3.02,;-2.03,2.25,;-3.37,3.02,;-3.37,4.56,;-4.7,2.25,;-4.7,.71,;-6.03,-.06,;-3.37,-.06,;-2.03,.71,;-.7,-.06,;-.7,-1.6,;.64,-2.37,;.64,-3.91,;-.7,-4.68,;-2.03,-3.91,;-2.03,-2.37,;-.7,-6.22,;.64,-6.99,;.64,-8.53,;1.97,-9.3,;.64,.71,;1.97,-.06,)| Show InChI InChI=1S/C22H27N5O4/c1-13-17-11-18(14-3-8-19(30-2)24-12-14)21(29)27(20(17)26-22(23)25-13)15-4-6-16(7-5-15)31-10-9-28/h3,8,11-12,15-16,28H,4-7,9-10H2,1-2H3,(H2,23,25,26)/t15-,16- | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Similars
| PDB
| n/a | n/a | 1.80 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Antagonist activity was determined against beta-1 adrenergic receptor in spontaneously beating rat atria |
Citation and Details
|
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit alpha isoform
(Homo sapiens (Human)) | BDBM50380313

(CHEMBL1234354 | US8633204, 286)Show SMILES COc1ccc(cn1)-c1cc2c(C)nc(N)nc2n([C@H]2CC[C@@H](CC2)OCCO)c1=O |r,wU:19.20,wD:22.27,(7.3,4.56,;5.97,5.33,;4.64,4.56,;3.3,5.33,;1.97,4.56,;1.97,3.02,;3.3,2.25,;4.64,3.02,;.64,2.25,;-.7,3.02,;-2.03,2.25,;-3.37,3.02,;-3.37,4.56,;-4.7,2.25,;-4.7,.71,;-6.03,-.06,;-3.37,-.06,;-2.03,.71,;-.7,-.06,;-.7,-1.6,;.64,-2.37,;.64,-3.91,;-.7,-4.68,;-2.03,-3.91,;-2.03,-2.37,;-.7,-6.22,;.64,-6.99,;.64,-8.53,;1.97,-9.3,;.64,.71,;1.97,-.06,)| Show InChI InChI=1S/C22H27N5O4/c1-13-17-11-18(14-3-8-19(30-2)24-12-14)21(29)27(20(17)26-22(23)25-13)15-4-6-16(7-5-15)31-10-9-28/h3,8,11-12,15-16,28H,4-7,9-10H2,1-2H3,(H2,23,25,26)/t15-,16- | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Similars
| PDB
| n/a | n/a | 1.80 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of choline acetyltransferase (ChAT) activity |
Citation and Details
|
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit gamma isoform
(Homo sapiens (Human)) | BDBM50380313

(CHEMBL1234354 | US8633204, 286)Show SMILES COc1ccc(cn1)-c1cc2c(C)nc(N)nc2n([C@H]2CC[C@@H](CC2)OCCO)c1=O |r,wU:19.20,wD:22.27,(7.3,4.56,;5.97,5.33,;4.64,4.56,;3.3,5.33,;1.97,4.56,;1.97,3.02,;3.3,2.25,;4.64,3.02,;.64,2.25,;-.7,3.02,;-2.03,2.25,;-3.37,3.02,;-3.37,4.56,;-4.7,2.25,;-4.7,.71,;-6.03,-.06,;-3.37,-.06,;-2.03,.71,;-.7,-.06,;-.7,-1.6,;.64,-2.37,;.64,-3.91,;-.7,-4.68,;-2.03,-3.91,;-2.03,-2.37,;-.7,-6.22,;.64,-6.99,;.64,-8.53,;1.97,-9.3,;.64,.71,;1.97,-.06,)| Show InChI InChI=1S/C22H27N5O4/c1-13-17-11-18(14-3-8-19(30-2)24-12-14)21(29)27(20(17)26-22(23)25-13)15-4-6-16(7-5-15)31-10-9-28/h3,8,11-12,15-16,28H,4-7,9-10H2,1-2H3,(H2,23,25,26)/t15-,16- | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Similars
| MMDB
PDB
| n/a | n/a | 1.90 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of squalene synthetase was determined in rat liver microsomes |
Citation and Details
|
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit gamma isoform
(Mus musculus (Mouse)) | BDBM50380313

(CHEMBL1234354 | US8633204, 286)Show SMILES COc1ccc(cn1)-c1cc2c(C)nc(N)nc2n([C@H]2CC[C@@H](CC2)OCCO)c1=O |r,wU:19.20,wD:22.27,(7.3,4.56,;5.97,5.33,;4.64,4.56,;3.3,5.33,;1.97,4.56,;1.97,3.02,;3.3,2.25,;4.64,3.02,;.64,2.25,;-.7,3.02,;-2.03,2.25,;-3.37,3.02,;-3.37,4.56,;-4.7,2.25,;-4.7,.71,;-6.03,-.06,;-3.37,-.06,;-2.03,.71,;-.7,-.06,;-.7,-1.6,;.64,-2.37,;.64,-3.91,;-.7,-4.68,;-2.03,-3.91,;-2.03,-2.37,;-.7,-6.22,;.64,-6.99,;.64,-8.53,;1.97,-9.3,;.64,.71,;1.97,-.06,)| Show InChI InChI=1S/C22H27N5O4/c1-13-17-11-18(14-3-8-19(30-2)24-12-14)21(29)27(20(17)26-22(23)25-13)15-4-6-16(7-5-15)31-10-9-28/h3,8,11-12,15-16,28H,4-7,9-10H2,1-2H3,(H2,23,25,26)/t15-,16- | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Similars
| PDB
| n/a | n/a | 1.90 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Antagonist activity was determined against beta-1 adrenergic receptor in spontaneously beating rat atria |
Citation and Details
|
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
RAC-beta serine/threonine-protein kinase
(Homo sapiens (Human)) | BDBM50502477

(ASB-183 | ASB183 | Afuresertib | GSK-2110183C | GS...)Show SMILES [H][C@@](CN)(Cc1cccc(F)c1)NC(=O)c1cc(c(Cl)s1)-c1c(Cl)cnn1C |r,wU:1.1,wD:1.0,(11.52,-5.94,;10.83,-4.68,;10.06,-6.01,;10.83,-7.35,;12.17,-3.88,;13.49,-4.64,;13.48,-6.2,;14.82,-6.95,;16.15,-6.19,;16.19,-4.64,;17.46,-3.84,;14.79,-3.91,;9.41,-3.84,;8.08,-4.6,;8.11,-6.13,;6.85,-3.92,;5.41,-4.51,;4.4,-3.4,;5.15,-2.04,;4.33,-.64,;6.62,-2.35,;2.85,-3.54,;1.85,-2.41,;2.36,-.88,;.42,-3.01,;.5,-4.52,;2.04,-4.83,;2.44,-6.32,)| Show InChI InChI=1S/C18H17Cl2FN4OS/c1-25-16(14(19)9-23-25)13-7-15(27-17(13)20)18(26)24-12(8-22)6-10-3-2-4-11(21)5-10/h2-5,7,9,12H,6,8,22H2,1H3,(H,24,26)/t12-/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
| | n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of choline acetyltransferase (ChAT) activity |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit beta isoform
(Mus musculus (Mouse)) | BDBM50380313

(CHEMBL1234354 | US8633204, 286)Show SMILES COc1ccc(cn1)-c1cc2c(C)nc(N)nc2n([C@H]2CC[C@@H](CC2)OCCO)c1=O |r,wU:19.20,wD:22.27,(7.3,4.56,;5.97,5.33,;4.64,4.56,;3.3,5.33,;1.97,4.56,;1.97,3.02,;3.3,2.25,;4.64,3.02,;.64,2.25,;-.7,3.02,;-2.03,2.25,;-3.37,3.02,;-3.37,4.56,;-4.7,2.25,;-4.7,.71,;-6.03,-.06,;-3.37,-.06,;-2.03,.71,;-.7,-.06,;-.7,-1.6,;.64,-2.37,;.64,-3.91,;-.7,-4.68,;-2.03,-3.91,;-2.03,-2.37,;-.7,-6.22,;.64,-6.99,;.64,-8.53,;1.97,-9.3,;.64,.71,;1.97,-.06,)| Show InChI InChI=1S/C22H27N5O4/c1-13-17-11-18(14-3-8-19(30-2)24-12-14)21(29)27(20(17)26-22(23)25-13)15-4-6-16(7-5-15)31-10-9-28/h3,8,11-12,15-16,28H,4-7,9-10H2,1-2H3,(H2,23,25,26)/t15-,16- | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Similars
| | n/a | n/a | 2.10 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Antagonist activity was determined against beta-1 adrenergic receptor in spontaneously beating rat atria |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit beta isoform
(Homo sapiens (Human)) | BDBM50380313

(CHEMBL1234354 | US8633204, 286)Show SMILES COc1ccc(cn1)-c1cc2c(C)nc(N)nc2n([C@H]2CC[C@@H](CC2)OCCO)c1=O |r,wU:19.20,wD:22.27,(7.3,4.56,;5.97,5.33,;4.64,4.56,;3.3,5.33,;1.97,4.56,;1.97,3.02,;3.3,2.25,;4.64,3.02,;.64,2.25,;-.7,3.02,;-2.03,2.25,;-3.37,3.02,;-3.37,4.56,;-4.7,2.25,;-4.7,.71,;-6.03,-.06,;-3.37,-.06,;-2.03,.71,;-.7,-.06,;-.7,-1.6,;.64,-2.37,;.64,-3.91,;-.7,-4.68,;-2.03,-3.91,;-2.03,-2.37,;-.7,-6.22,;.64,-6.99,;.64,-8.53,;1.97,-9.3,;.64,.71,;1.97,-.06,)| Show InChI InChI=1S/C22H27N5O4/c1-13-17-11-18(14-3-8-19(30-2)24-12-14)21(29)27(20(17)26-22(23)25-13)15-4-6-16(7-5-15)31-10-9-28/h3,8,11-12,15-16,28H,4-7,9-10H2,1-2H3,(H2,23,25,26)/t15-,16- | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Similars
| | n/a | n/a | 2.10 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of choline acetyltransferase (ChAT) activity |
Citation and Details
|
More data for this
Ligand-Target Pair | |
RAC-gamma serine/threonine-protein kinase
(Homo sapiens (Human)) | BDBM50502477

(ASB-183 | ASB183 | Afuresertib | GSK-2110183C | GS...)Show SMILES [H][C@@](CN)(Cc1cccc(F)c1)NC(=O)c1cc(c(Cl)s1)-c1c(Cl)cnn1C |r,wU:1.1,wD:1.0,(11.52,-5.94,;10.83,-4.68,;10.06,-6.01,;10.83,-7.35,;12.17,-3.88,;13.49,-4.64,;13.48,-6.2,;14.82,-6.95,;16.15,-6.19,;16.19,-4.64,;17.46,-3.84,;14.79,-3.91,;9.41,-3.84,;8.08,-4.6,;8.11,-6.13,;6.85,-3.92,;5.41,-4.51,;4.4,-3.4,;5.15,-2.04,;4.33,-.64,;6.62,-2.35,;2.85,-3.54,;1.85,-2.41,;2.36,-.88,;.42,-3.01,;.5,-4.52,;2.04,-4.83,;2.44,-6.32,)| Show InChI InChI=1S/C18H17Cl2FN4OS/c1-25-16(14(19)9-23-25)13-7-15(27-17(13)20)18(26)24-12(8-22)6-10-3-2-4-11(21)5-10/h2-5,7,9,12H,6,8,22H2,1H3,(H,24,26)/t12-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
| | n/a | n/a | 2.60 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of acetylcholinesterase (AChE) activity |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Polyamine deacetylase HDAC10
(Homo sapiens (Human)) | BDBM50405632

(CHEMBL5278968)Show InChI InChI=1S/C16H21NO2/c1-12(2)17-10-15(18)11-19-16-8-7-13-5-3-4-6-14(13)9-16/h3-9,12,15,17-18H,10-11H2,1-2H3 | NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Similars
| | n/a | n/a | 2.80 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of choline acetyltransferase (ChAT) activity |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit alpha isoform
(Homo sapiens (Human)) | BDBM50405637
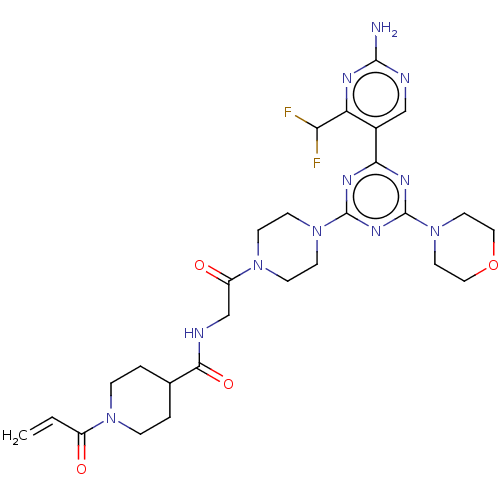
(CHEMBL5273641)Show InChI InChI=1S/C16H25NO2/c18-16(15-10-4-5-11-17-15)13-19-12-6-9-14-7-2-1-3-8-14/h1-3,7-8,15-18H,4-6,9-13H2/t15-,16-/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
PDB
UniChem
Similars
| PDB
| n/a | n/a | 2.90 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of acetylcholinesterase (AChE) activity |
Citation and Details
|
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
RAC-alpha serine/threonine-protein kinase
(Homo sapiens (Human)) | BDBM50405631
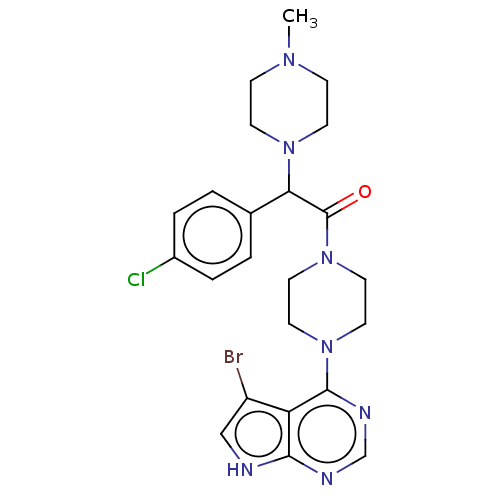
(CHEMBL5286570)Show InChI InChI=1S/C14H21NO2/c1-15-10-6-5-9-13(15)14(16)11-17-12-7-3-2-4-8-12/h2-4,7-8,13-14,16H,5-6,9-11H2,1H3/t13-,14+/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| | n/a | n/a | 3.40 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Cardioselectivity for the beta-1 adrenergic receptor was determined against isoprenaline (antagonism) in isolated rat atria |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit alpha isoform
(Homo sapiens (Human)) | BDBM92862

(US9284315, BEZ-235 | mTOR Inhibitor, BEZ235)Show SMILES Cn1c2cnc3ccc(cc3c2n(-c2ccc(cc2)C(C)(C)C#N)c1=O)-c1cnc2ccccc2c1 Show InChI InChI=1S/C30H23N5O/c1-30(2,18-31)22-9-11-23(12-10-22)35-28-24-15-19(21-14-20-6-4-5-7-25(20)32-16-21)8-13-26(24)33-17-27(28)34(3)29(35)36/h4-17H,1-3H3 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
Similars
| | n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of choline acetyltransferase (ChAT) activity |
Citation and Details
|
More data for this
Ligand-Target Pair | |
RAC-beta serine/threonine-protein kinase
(Homo sapiens (Human)) | BDBM50593633
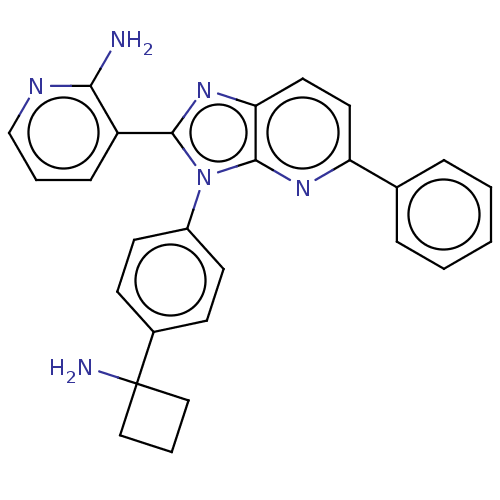
(ARQ 092 | ARQ 092 FREE BASE | ARQ-092 | Arq-092 | ...)Show SMILES Nc1ncccc1-c1nc2ccc(nc2n1-c1ccc(cc1)C1(N)CCC1)-c1ccccc1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
PDB
UniChem
| | n/a | n/a | 4.5 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of choline acetyltransferase (ChAT) activity |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit delta isoform
(Homo sapiens (Human)) | BDBM50315213
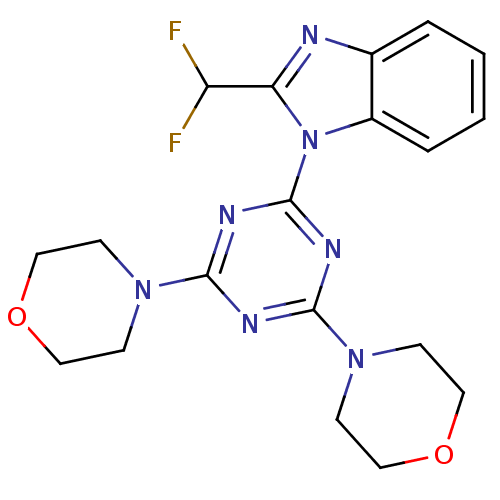
(2-(difluoromethyl)-1-(4,6-dimorpholin-4-yl-1,3,5-t...)Show SMILES FC(F)c1nc2ccccc2n1-c1nc(nc(n1)N1CCOCC1)N1CCOCC1 Show InChI InChI=1S/C19H21F2N7O2/c20-15(21)16-22-13-3-1-2-4-14(13)28(16)19-24-17(26-5-9-29-10-6-26)23-18(25-19)27-7-11-30-12-8-27/h1-4,15H,5-12H2 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| MMDB
PDB
| n/a | n/a | 4.60 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of acetylcholinesterase (AChE) activity |
Citation and Details
|
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
RAC-gamma serine/threonine-protein kinase
(Homo sapiens (Human)) | BDBM50405631

(CHEMBL5286570)Show InChI InChI=1S/C14H21NO2/c1-15-10-6-5-9-13(15)14(16)11-17-12-7-3-2-4-8-12/h2-4,7-8,13-14,16H,5-6,9-11H2,1H3/t13-,14+/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| | n/a | n/a | 4.70 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Antagonist activity was determined against beta-1 adrenergic receptor in spontaneously beating rat atria |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Histone deacetylase 2
(Homo sapiens (Human)) | BDBM50405632

(CHEMBL5278968)Show InChI InChI=1S/C16H21NO2/c1-12(2)17-10-15(18)11-19-16-8-7-13-5-3-4-6-14(13)9-16/h3-9,12,15,17-18H,10-11H2,1-2H3 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Similars
| | n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of acetylcholinesterase (AChE) activity |
Citation and Details
|
More data for this
Ligand-Target Pair | |
RAC-alpha serine/threonine-protein kinase
(Homo sapiens (Human)) | BDBM50593633

(ARQ 092 | ARQ 092 FREE BASE | ARQ-092 | Arq-092 | ...)Show SMILES Nc1ncccc1-c1nc2ccc(nc2n1-c1ccc(cc1)C1(N)CCC1)-c1ccccc1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
PDB
UniChem
| PDB
| n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of acetylcholinesterase (AChE) activity |
Citation and Details
|
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit gamma isoform
(Homo sapiens (Human)) | BDBM92862

(US9284315, BEZ-235 | mTOR Inhibitor, BEZ235)Show SMILES Cn1c2cnc3ccc(cc3c2n(-c2ccc(cc2)C(C)(C)C#N)c1=O)-c1cnc2ccccc2c1 Show InChI InChI=1S/C30H23N5O/c1-30(2,18-31)22-9-11-23(12-10-22)35-28-24-15-19(21-14-20-6-4-5-7-25(20)32-16-21)8-13-26(24)33-17-27(28)34(3)29(35)36/h4-17H,1-3H3 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
Similars
| | n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of acetylcholinesterase (AChE) activity |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit alpha isoform
(Homo sapiens (Human)) | BDBM50405640
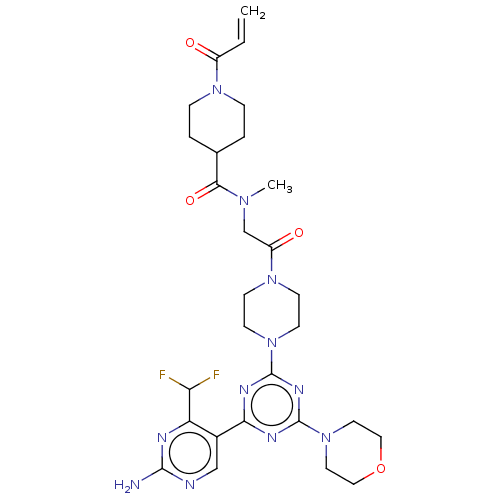
(CHEMBL5268290)Show SMILES Nc1nc2ccc(COc3ccc(cc3)C(=O)NC(CCC(O)=O)C(O)=O)cc2c(=O)[nH]1 Show InChI InChI=1S/C21H20N4O7/c22-21-24-15-6-1-11(9-14(15)19(29)25-21)10-32-13-4-2-12(3-5-13)18(28)23-16(20(30)31)7-8-17(26)27/h1-6,9,16H,7-8,10H2,(H,23,28)(H,26,27)(H,30,31)(H3,22,24,25,29) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
PDB
UniChem
Similars
| PDB
| n/a | n/a | 5.10 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of choline acetyltransferase (ChAT) activity |
Citation and Details
|
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit beta isoform
(Homo sapiens (Human)) | BDBM50059637
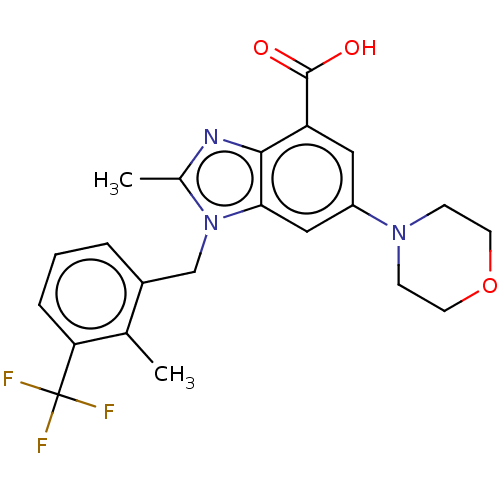
(GSK-2636771 | GSK2636771 | US10660898, Example 31)Show SMILES Cc1nc2c(cc(cc2n1Cc1cccc(c1C)C(F)(F)F)N1CCOCC1)C(O)=O Show InChI InChI=1S/C22H22F3N3O3/c1-13-15(4-3-5-18(13)22(23,24)25)12-28-14(2)26-20-17(21(29)30)10-16(11-19(20)28)27-6-8-31-9-7-27/h3-5,10-11H,6-9,12H2,1-2H3,(H,29,30) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
Similars
| | n/a | n/a | 5.20 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of choline acetyltransferase (ChAT) activity |
Citation and Details
|
More data for this
Ligand-Target Pair | |
RAC-alpha serine/threonine-protein kinase
(Homo sapiens (Human)) | BDBM312517
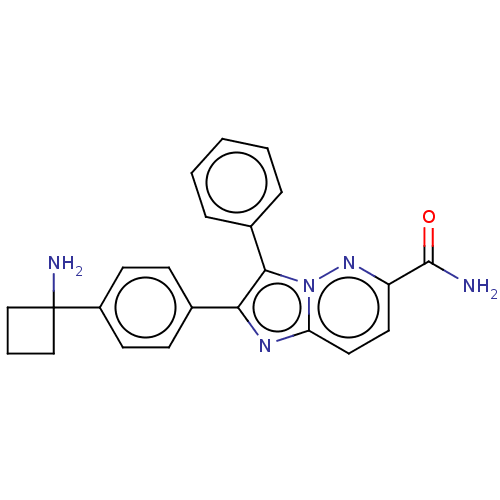
(2-[4-(1-Aminocyclobutyl)phenyl]-3-phenylimidazo[1,...)Show SMILES NC(=O)c1ccc2nc(c(-c3ccccc3)n2n1)-c1ccc(cc1)C1(N)CCC1 Show InChI InChI=1S/C23H21N5O/c24-22(29)18-11-12-19-26-20(21(28(19)27-18)16-5-2-1-3-6-16)15-7-9-17(10-8-15)23(25)13-4-14-23/h1-3,5-12H,4,13-14,25H2,(H2,24,29) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
| | n/a | n/a | 5.20 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of acetylcholinesterase (AChE) activity |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Histone deacetylase 11
(Homo sapiens (Human)) | BDBM50405632

(CHEMBL5278968)Show InChI InChI=1S/C16H21NO2/c1-12(2)17-10-15(18)11-19-16-8-7-13-5-3-4-6-14(13)9-16/h3-9,12,15,17-18H,10-11H2,1-2H3 | NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Similars
| | n/a | n/a | 5.40 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of choline acetyltransferase (ChAT) activity |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase mTOR
(Homo sapiens (Human)) | BDBM92862

(US9284315, BEZ-235 | mTOR Inhibitor, BEZ235)Show SMILES Cn1c2cnc3ccc(cc3c2n(-c2ccc(cc2)C(C)(C)C#N)c1=O)-c1cnc2ccccc2c1 Show InChI InChI=1S/C30H23N5O/c1-30(2,18-31)22-9-11-23(12-10-22)35-28-24-15-19(21-14-20-6-4-5-7-25(20)32-16-21)8-13-26(24)33-17-27(28)34(3)29(35)36/h4-17H,1-3H3 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
Similars
| | n/a | n/a | 6 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of acetylcholinesterase (AChE) activity |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Histone deacetylase 6
(Homo sapiens (Human)) | BDBM50503943

(CHEMBL4536105)Show SMILES COc1ncc(cc1F)-c1cc(OCCCCCCC(=O)NO)c2ncnc(C)c2c1 Show InChI InChI=1S/C22H25FN4O4/c1-14-17-9-15(16-10-18(23)22(30-2)24-12-16)11-19(21(17)26-13-25-14)31-8-6-4-3-5-7-20(28)27-29/h9-13,29H,3-8H2,1-2H3,(H,27,28) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
| | n/a | n/a | 6.60 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of choline acetyltransferase (ChAT) activity |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit alpha isoform
(Homo sapiens (Human)) | BDBM50426176
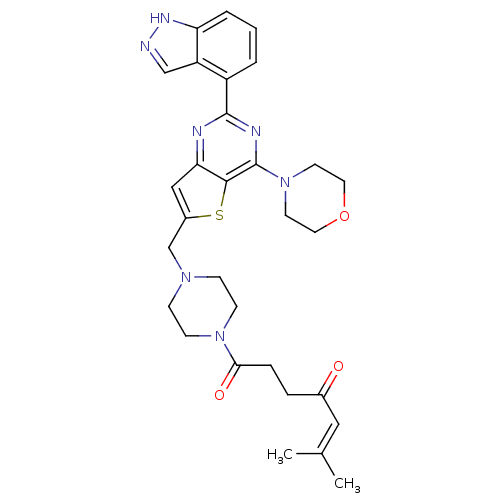
(CHEMBL2316958)Show SMILES CC(C)=CC(=O)CCC(=O)N1CCN(Cc2cc3nc(nc(N4CCOCC4)c3s2)-c2cccc3[nH]ncc23)CC1 |(2.18,-16.72,;2.94,-15.38,;2.16,-14.06,;4.48,-15.37,;5.24,-14.03,;4.46,-12.71,;6.78,-14.02,;7.54,-12.68,;9.08,-12.67,;9.86,-14,;9.84,-11.33,;11.38,-11.32,;12.14,-9.98,;11.37,-8.66,;12.13,-7.32,;13.67,-7.31,;14.58,-8.56,;16.04,-8.08,;17.38,-8.84,;18.71,-8.06,;18.69,-6.52,;17.36,-5.76,;17.35,-4.23,;18.68,-3.45,;18.67,-1.92,;17.33,-1.15,;16,-1.93,;16.01,-3.47,;16.04,-6.54,;14.57,-6.07,;20.04,-8.82,;20.05,-10.36,;21.39,-11.12,;22.72,-10.34,;22.7,-8.79,;23.82,-7.76,;23.19,-6.37,;21.67,-6.54,;21.36,-8.04,;9.83,-8.66,;9.07,-10,)| Show InChI InChI=1S/C30H35N7O3S/c1-20(2)16-21(38)6-7-27(39)36-10-8-35(9-11-36)19-22-17-26-28(41-22)30(37-12-14-40-15-13-37)33-29(32-26)23-4-3-5-25-24(23)18-31-34-25/h3-5,16-18H,6-15,19H2,1-2H3,(H,31,34) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
PDB
UniChem
Similars
| PDB
| n/a | n/a | 6.80 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of acetylcholinesterase (AChE) activity |
Citation and Details
|
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit delta isoform
(Homo sapiens (Human)) | BDBM92862

(US9284315, BEZ-235 | mTOR Inhibitor, BEZ235)Show SMILES Cn1c2cnc3ccc(cc3c2n(-c2ccc(cc2)C(C)(C)C#N)c1=O)-c1cnc2ccccc2c1 Show InChI InChI=1S/C30H23N5O/c1-30(2,18-31)22-9-11-23(12-10-22)35-28-24-15-19(21-14-20-6-4-5-7-25(20)32-16-21)8-13-26(24)33-17-27(28)34(3)29(35)36/h4-17H,1-3H3 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
Similars
| | n/a | n/a | 7 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of acetylcholinesterase (AChE) activity |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit delta isoform
(Homo sapiens (Human)) | BDBM50405641
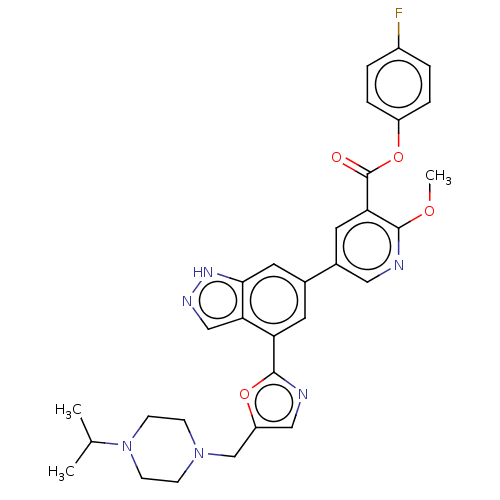
(CHEMBL5286800)Show SMILES CN(Cc1ccc(cc1)C(=O)NC(CCC(O)=O)C(O)=O)c1ccc2nc(N)[nH]c(=O)c2c1C Show InChI InChI=1S/C23H25N5O6/c1-12-17(9-7-15-19(12)21(32)27-23(24)26-15)28(2)11-13-3-5-14(6-4-13)20(31)25-16(22(33)34)8-10-18(29)30/h3-7,9,16H,8,10-11H2,1-2H3,(H,25,31)(H,29,30)(H,33,34)(H3,24,26,27,32) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| | n/a | n/a | 7.90 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of acetylcholinesterase (AChE) activity |
Citation and Details
|
More data for this
Ligand-Target Pair | |
RAC-alpha serine/threonine-protein kinase
(Homo sapiens (Human)) | BDBM50313650
(8-(4-(1-aminocyclobutyl)phenyl)-9-phenyl-[1,2,4]tr...)Show SMILES NC1(CCC1)c1ccc(cc1)-c1nc2ccn3c(n[nH]c3=O)c2cc1-c1ccccc1 Show InChI InChI=1S/C25H21N5O/c26-25(12-4-13-25)18-9-7-17(8-10-18)22-19(16-5-2-1-3-6-16)15-20-21(27-22)11-14-30-23(20)28-29-24(30)31/h1-3,5-11,14-15H,4,12-13,26H2,(H,29,31) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
| | n/a | n/a | 8 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of choline acetyltransferase (ChAT) activity |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit delta isoform
(Homo sapiens (Human)) | BDBM50503943

(CHEMBL4536105)Show SMILES COc1ncc(cc1F)-c1cc(OCCCCCCC(=O)NO)c2ncnc(C)c2c1 Show InChI InChI=1S/C22H25FN4O4/c1-14-17-9-15(16-10-18(23)22(30-2)24-12-16)11-19(21(17)26-13-25-14)31-8-6-4-3-5-7-20(28)27-29/h9-13,29H,3-8H2,1-2H3,(H,27,28) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
| | n/a | n/a | 8.10 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Antagonist activity was determined against beta-1 adrenergic receptor in spontaneously beating rat atria |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit alpha isoform
(Homo sapiens (Human)) | BDBM50405629

(CHEMBL5278435)Show InChI InChI=1S/C14H21NO2/c16-14(13-8-4-5-9-15-13)11-17-10-12-6-2-1-3-7-12/h1-3,6-7,13-16H,4-5,8-11H2/t13-,14+/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| | n/a | n/a | 12 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Antagonist activity was determined against beta-1 adrenergic receptor in spontaneously beating rat atria |
Citation and Details
|
More data for this
Ligand-Target Pair | |
RAC-beta serine/threonine-protein kinase
(Homo sapiens (Human)) | BDBM50313650

(8-(4-(1-aminocyclobutyl)phenyl)-9-phenyl-[1,2,4]tr...)Show SMILES NC1(CCC1)c1ccc(cc1)-c1nc2ccn3c(n[nH]c3=O)c2cc1-c1ccccc1 Show InChI InChI=1S/C25H21N5O/c26-25(12-4-13-25)18-9-7-17(8-10-18)22-19(16-5-2-1-3-6-16)15-20-21(27-22)11-14-30-23(20)28-29-24(30)31/h1-3,5-11,14-15H,4,12-13,26H2,(H,29,31) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
| | n/a | n/a | 12 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of acetylcholinesterase (AChE) activity |
Citation and Details
|
More data for this
Ligand-Target Pair | |
RAC-gamma serine/threonine-protein kinase
(Homo sapiens (Human)) | BDBM50593633

(ARQ 092 | ARQ 092 FREE BASE | ARQ-092 | Arq-092 | ...)Show SMILES Nc1ncccc1-c1nc2ccc(nc2n1-c1ccc(cc1)C1(N)CCC1)-c1ccccc1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
PDB
UniChem
| | n/a | n/a | 16 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of choline acetyltransferase (ChAT) activity |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit alpha isoform
(Homo sapiens (Human)) | BDBM50315213

(2-(difluoromethyl)-1-(4,6-dimorpholin-4-yl-1,3,5-t...)Show SMILES FC(F)c1nc2ccccc2n1-c1nc(nc(n1)N1CCOCC1)N1CCOCC1 Show InChI InChI=1S/C19H21F2N7O2/c20-15(21)16-22-13-3-1-2-4-14(13)28(16)19-24-17(26-5-9-29-10-6-26)23-18(25-19)27-7-11-30-12-8-27/h1-4,15H,5-12H2 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| | n/a | n/a | 16 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of acetylcholinesterase (AChE) activity |
Citation and Details
|
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data