

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Dihydrofolate reductase (Homo sapiens (Human)) | BDBM18050 (2-[(4-{[(2,4-diaminopteridin-6-yl)methyl](methyl)a...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | DrugBank MMDB PDB PubMed | n/a | n/a | 0.700 | n/a | n/a | n/a | n/a | n/a | n/a |
Duquesne University Curated by ChEMBL | Assay Description Inhibitory concentration of the compound against CCRF-CEM leukemic cell DHFR(Dihydro folate reductase). | J Med Chem 38: 3798-805 (1995) BindingDB Entry DOI: 10.7270/Q2FQ9VN5 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Dihydrofolate reductase (Lactobacillus casei) | BDBM18050 (2-[(4-{[(2,4-diaminopteridin-6-yl)methyl](methyl)a...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | PDB PubMed | n/a | n/a | 6 | n/a | n/a | n/a | n/a | n/a | n/a |
Duquesne University Curated by ChEMBL | Assay Description inhibitory concentration of the compound against Lactobacillus casei DHFR(Dihydro folate reductase). | J Med Chem 38: 3798-805 (1995) BindingDB Entry DOI: 10.7270/Q2FQ9VN5 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Dihydrofolate reductase (Homo sapiens (Human)) | BDBM18050 (2-[(4-{[(2,4-diaminopteridin-6-yl)methyl](methyl)a...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | DrugBank MMDB PDB PubMed | n/a | n/a | 40 | n/a | n/a | n/a | n/a | n/a | n/a |
Duquesne University Curated by ChEMBL | Assay Description Inhibitory concentration of the compound against recombinant human DHFR(Dihydro folate reductase). | J Med Chem 38: 3798-805 (1995) BindingDB Entry DOI: 10.7270/Q2FQ9VN5 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Dihydrofolate reductase (Homo sapiens (Human)) | BDBM50030819 ((S)-2-(4-(2-(2,4-diaminofuro[2,3-d]pyrimidin-5-yl)...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 100 | n/a | n/a | n/a | n/a | n/a | n/a |
Duquesne University Curated by ChEMBL | Assay Description Inhibitory concentration of the compound against Lactobacillus casei DHFR(Dihydro folate reductase). | J Med Chem 38: 3798-805 (1995) BindingDB Entry DOI: 10.7270/Q2FQ9VN5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dihydrofolate reductase (Lactobacillus casei) | BDBM50030819 ((S)-2-(4-(2-(2,4-diaminofuro[2,3-d]pyrimidin-5-yl)...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 100 | n/a | n/a | n/a | n/a | n/a | n/a |
Duquesne University Curated by ChEMBL | Assay Description Inhibitory concentration of the compound against Lactobacillus casei DHFR(Dihydro folate reductase). | J Med Chem 38: 3798-805 (1995) BindingDB Entry DOI: 10.7270/Q2FQ9VN5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dihydrofolate reductase (Homo sapiens (Human)) | BDBM50030820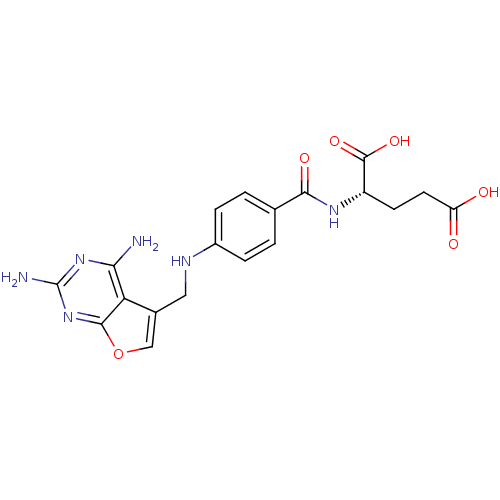 ((S)-2-(4-((2,4-diaminofuro[2,3-d]pyrimidin-5-yl)me...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 450 | n/a | n/a | n/a | n/a | n/a | n/a |
Duquesne University Curated by ChEMBL | Assay Description Inhibitory concentration of the compound against recombinant human DHFR(Dihydro folate reductase). | J Med Chem 38: 3798-805 (1995) BindingDB Entry DOI: 10.7270/Q2FQ9VN5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dihydrofolate reductase (Lactobacillus casei) | BDBM50030820 ((S)-2-(4-((2,4-diaminofuro[2,3-d]pyrimidin-5-yl)me...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 560 | n/a | n/a | n/a | n/a | n/a | n/a |
Duquesne University Curated by ChEMBL | Assay Description inhibitory concentration of the compound against Lactobacillus casei DHFR(Dihydro folate reductase). | J Med Chem 38: 3798-805 (1995) BindingDB Entry DOI: 10.7270/Q2FQ9VN5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dihydrofolate reductase (Homo sapiens (Human)) | BDBM50030819 ((S)-2-(4-(2-(2,4-diaminofuro[2,3-d]pyrimidin-5-yl)...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Duquesne University Curated by ChEMBL | Assay Description Inhibitory concentration of the compound against recombinant human DHFR(Dihydro folate reductase). | J Med Chem 38: 3798-805 (1995) BindingDB Entry DOI: 10.7270/Q2FQ9VN5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dihydrofolate reductase (Homo sapiens (Human)) | BDBM50030818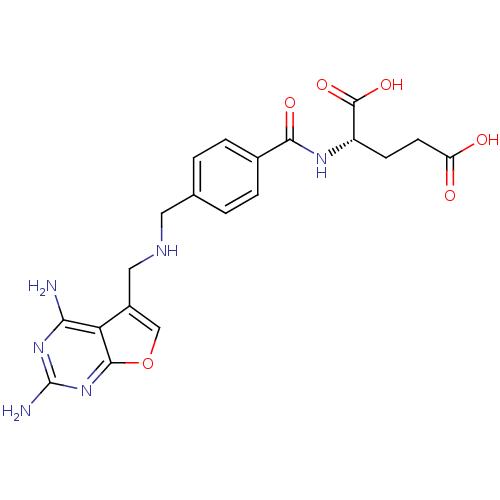 ((S)-2-(4-{[(2,4-Diamino-furo[2,3-d]pyrimidin-5-ylm...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 3.05E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Duquesne University Curated by ChEMBL | Assay Description Inhibitory concentration of the compound against CCRF-CEM leukemic cell DHFR(Dihydro folate reductase). | J Med Chem 38: 3798-805 (1995) BindingDB Entry DOI: 10.7270/Q2FQ9VN5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Thymidylate synthase (Homo sapiens (Human)) | BDBM18050 (2-[(4-{[(2,4-diaminopteridin-6-yl)methyl](methyl)a...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | DrugBank PDB PubMed | n/a | n/a | 1.70E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Duquesne University Curated by ChEMBL | Assay Description Inhibitory concentration of the compound against recombinant human TS (Thymidylate synthase). | J Med Chem 38: 3798-805 (1995) BindingDB Entry DOI: 10.7270/Q2FQ9VN5 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Thymidylate synthase (Homo sapiens (Human)) | BDBM50030820 ((S)-2-(4-((2,4-diaminofuro[2,3-d]pyrimidin-5-yl)me...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | >2.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Duquesne University Curated by ChEMBL | Assay Description Inhibitory concentration of the compound against Lactobacillus casei TS (Thymidylate synthase). | J Med Chem 38: 3798-805 (1995) BindingDB Entry DOI: 10.7270/Q2FQ9VN5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Thymidylate synthase (Homo sapiens (Human)) | BDBM50030818 ((S)-2-(4-{[(2,4-Diamino-furo[2,3-d]pyrimidin-5-ylm...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | >2.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Duquesne University Curated by ChEMBL | Assay Description Inhibitory concentration of the compound against Lactobacillus casei TS (Thymidylate synthase). | J Med Chem 38: 3798-805 (1995) BindingDB Entry DOI: 10.7270/Q2FQ9VN5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Thymidylate synthase (Lactobacillus casei) | BDBM50030819 ((S)-2-(4-(2-(2,4-diaminofuro[2,3-d]pyrimidin-5-yl)...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 2.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Duquesne University Curated by ChEMBL | Assay Description Inhibitory concentration of the compound against Lactobacillus casei TS (Thymidylate synthase). | J Med Chem 38: 3798-805 (1995) BindingDB Entry DOI: 10.7270/Q2FQ9VN5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dihydrofolate reductase (Homo sapiens (Human)) | BDBM50030818 ((S)-2-(4-{[(2,4-Diamino-furo[2,3-d]pyrimidin-5-ylm...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | >2.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Duquesne University Curated by ChEMBL | Assay Description Inhibitory concentration of the compound against recombinant human DHFR(Dihydro folate reductase). | J Med Chem 38: 3798-805 (1995) BindingDB Entry DOI: 10.7270/Q2FQ9VN5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Thymidylate synthase (Lactobacillus casei) | BDBM50030820 ((S)-2-(4-((2,4-diaminofuro[2,3-d]pyrimidin-5-yl)me...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | >2.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Duquesne University Curated by ChEMBL | Assay Description Inhibitory concentration of the compound against Lactobacillus casei TS (Thymidylate synthase). | J Med Chem 38: 3798-805 (1995) BindingDB Entry DOI: 10.7270/Q2FQ9VN5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Thymidylate synthase (Lactobacillus casei) | BDBM50030818 ((S)-2-(4-{[(2,4-Diamino-furo[2,3-d]pyrimidin-5-ylm...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | >2.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Duquesne University Curated by ChEMBL | Assay Description Inhibitory concentration of the compound against Lactobacillus casei TS (Thymidylate synthase). | J Med Chem 38: 3798-805 (1995) BindingDB Entry DOI: 10.7270/Q2FQ9VN5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dihydrofolate reductase (Lactobacillus casei) | BDBM50030818 ((S)-2-(4-{[(2,4-Diamino-furo[2,3-d]pyrimidin-5-ylm...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | >2.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Duquesne University Curated by ChEMBL | Assay Description inhibitory concentration of the compound against Lactobacillus casei DHFR(Dihydro folate reductase). | J Med Chem 38: 3798-805 (1995) BindingDB Entry DOI: 10.7270/Q2FQ9VN5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Thymidylate synthase (Homo sapiens (Human)) | BDBM50030819 ((S)-2-(4-(2-(2,4-diaminofuro[2,3-d]pyrimidin-5-yl)...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 2.20E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Duquesne University Curated by ChEMBL | Assay Description Inhibitory concentration of the compound against recombinant human TS (Thymidylate synthase) | J Med Chem 38: 3798-805 (1995) BindingDB Entry DOI: 10.7270/Q2FQ9VN5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||