

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Cholinesterase (Equus caballus (Horse)) | BDBM50430730 (CHEMBL2333926) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
Sun Yat-sen University Curated by ChEMBL | Assay Description Inhibition of horse serum BChE using butylthiocholine chloride as substrate after 15 mins by Ellman's method | Eur J Med Chem 62: 745-53 (2013) Article DOI: 10.1016/j.ejmech.2013.01.039 BindingDB Entry DOI: 10.7270/Q2C82BN6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Amine oxidase [flavin-containing] A (Homo sapiens (Human)) | BDBM15581 (CHEMBL8706 | CLG | CLORGILINE | Clorgyline | N-[3-...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | n/a | n/a | 4.10 | n/a | n/a | n/a | n/a | n/a | n/a |
Sun Yat-sen University Curated by ChEMBL | Assay Description Inhibition of recombinant human MAO-A using p-tyramine substrate preincubated for 15 mins before substrate addition measured after 20 mins by fluores... | Eur J Med Chem 62: 745-53 (2013) Article DOI: 10.1016/j.ejmech.2013.01.039 BindingDB Entry DOI: 10.7270/Q2C82BN6 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Cholinesterase (Equus caballus (Horse)) | BDBM50430731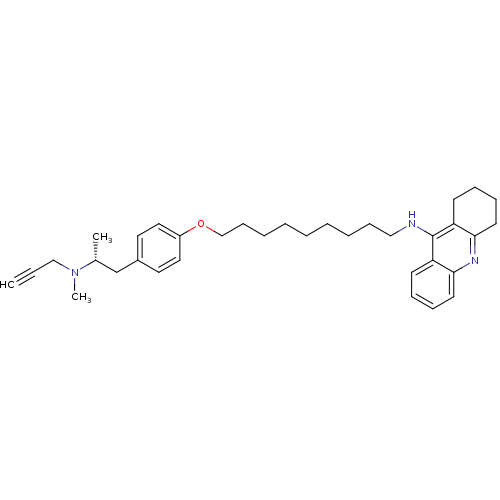 (CHEMBL2333925) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 9.40 | n/a | n/a | n/a | n/a | n/a | n/a |
Sun Yat-sen University Curated by ChEMBL | Assay Description Inhibition of horse serum BChE using butylthiocholine chloride as substrate after 15 mins by Ellman's method | Eur J Med Chem 62: 745-53 (2013) Article DOI: 10.1016/j.ejmech.2013.01.039 BindingDB Entry DOI: 10.7270/Q2C82BN6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM50430729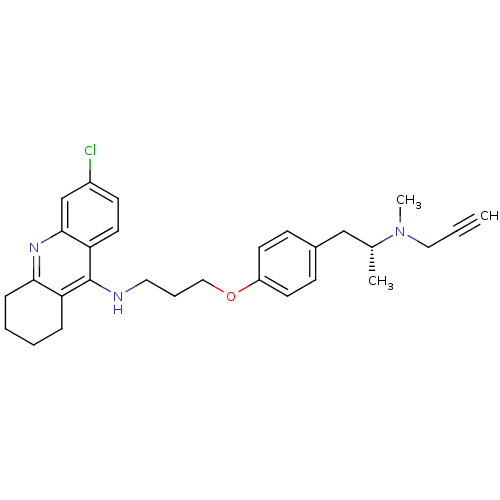 (CHEMBL2333927) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 14 | n/a | n/a | n/a | n/a | n/a | n/a |
Sun Yat-sen University Curated by ChEMBL | Assay Description Inhibition of electric eel AChE using acetylthiocholine chloride as substrate after 15 mins by Ellman's method | Eur J Med Chem 62: 745-53 (2013) Article DOI: 10.1016/j.ejmech.2013.01.039 BindingDB Entry DOI: 10.7270/Q2C82BN6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Equus caballus (Horse)) | BDBM50430733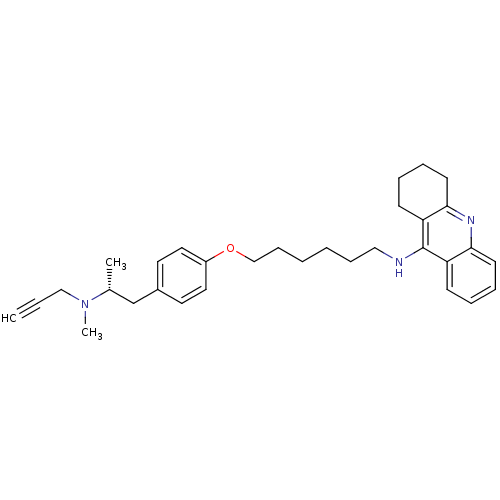 (CHEMBL2333936) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 16 | n/a | n/a | n/a | n/a | n/a | n/a |
Sun Yat-sen University Curated by ChEMBL | Assay Description Inhibition of horse serum BChE using butylthiocholine chloride as substrate after 15 mins by Ellman's method | Eur J Med Chem 62: 745-53 (2013) Article DOI: 10.1016/j.ejmech.2013.01.039 BindingDB Entry DOI: 10.7270/Q2C82BN6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Equus caballus (Horse)) | BDBM50430726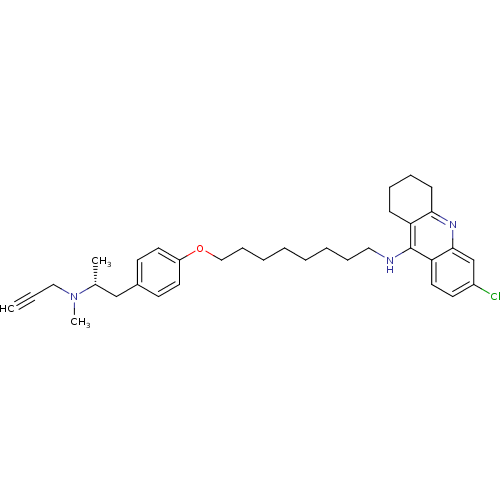 (CHEMBL2333930) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 18 | n/a | n/a | n/a | n/a | n/a | n/a |
Sun Yat-sen University Curated by ChEMBL | Assay Description Inhibition of horse serum BChE using butylthiocholine chloride as substrate after 15 mins by Ellman's method | Eur J Med Chem 62: 745-53 (2013) Article DOI: 10.1016/j.ejmech.2013.01.039 BindingDB Entry DOI: 10.7270/Q2C82BN6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Equus caballus (Horse)) | BDBM50430727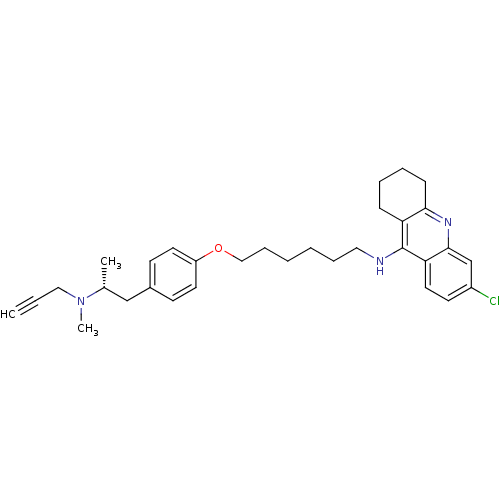 (CHEMBL2333929) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 19 | n/a | n/a | n/a | n/a | n/a | n/a |
Sun Yat-sen University Curated by ChEMBL | Assay Description Inhibition of horse serum BChE using butylthiocholine chloride as substrate after 15 mins by Ellman's method | Eur J Med Chem 62: 745-53 (2013) Article DOI: 10.1016/j.ejmech.2013.01.039 BindingDB Entry DOI: 10.7270/Q2C82BN6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Equus caballus (Horse)) | BDBM50430728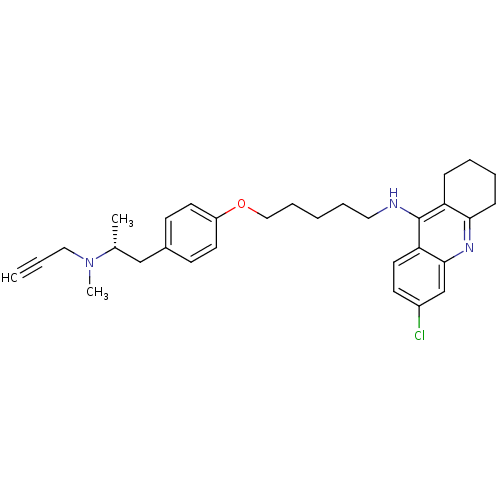 (CHEMBL2333928) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 19 | n/a | n/a | n/a | n/a | n/a | n/a |
Sun Yat-sen University Curated by ChEMBL | Assay Description Inhibition of horse serum BChE using butylthiocholine chloride as substrate after 15 mins by Ellman's method | Eur J Med Chem 62: 745-53 (2013) Article DOI: 10.1016/j.ejmech.2013.01.039 BindingDB Entry DOI: 10.7270/Q2C82BN6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Equus caballus (Horse)) | BDBM8961 (1,2,3,4-tetrahydro-9-acridinamine | 1,2,3,4-tetrah...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | n/a | n/a | 22 | n/a | n/a | n/a | n/a | n/a | n/a |
Sun Yat-sen University Curated by ChEMBL | Assay Description Inhibition of horse serum BChE using butylthiocholine chloride as substrate after 15 mins by Ellman's method | Eur J Med Chem 62: 745-53 (2013) Article DOI: 10.1016/j.ejmech.2013.01.039 BindingDB Entry DOI: 10.7270/Q2C82BN6 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM50430731 (CHEMBL2333925) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 23 | n/a | n/a | n/a | n/a | n/a | n/a |
Sun Yat-sen University Curated by ChEMBL | Assay Description Inhibition of electric eel AChE using acetylthiocholine chloride as substrate after 15 mins by Ellman's method | Eur J Med Chem 62: 745-53 (2013) Article DOI: 10.1016/j.ejmech.2013.01.039 BindingDB Entry DOI: 10.7270/Q2C82BN6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM50430730 (CHEMBL2333926) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 23 | n/a | n/a | n/a | n/a | n/a | n/a |
Sun Yat-sen University Curated by ChEMBL | Assay Description Inhibition of electric eel AChE using acetylthiocholine chloride as substrate after 15 mins by Ellman's method | Eur J Med Chem 62: 745-53 (2013) Article DOI: 10.1016/j.ejmech.2013.01.039 BindingDB Entry DOI: 10.7270/Q2C82BN6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Equus caballus (Horse)) | BDBM50430734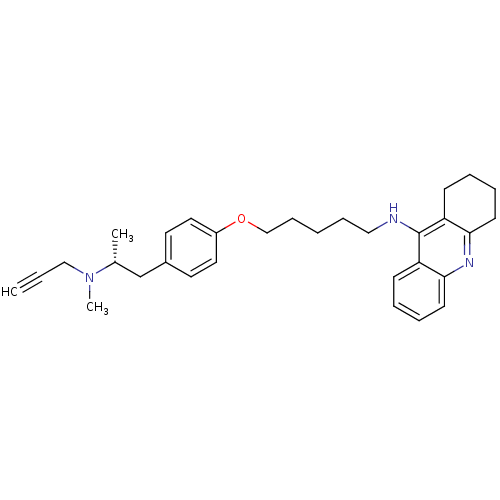 (CHEMBL2333935) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 24 | n/a | n/a | n/a | n/a | n/a | n/a |
Sun Yat-sen University Curated by ChEMBL | Assay Description Inhibition of horse serum BChE using butylthiocholine chloride as substrate after 15 mins by Ellman's method | Eur J Med Chem 62: 745-53 (2013) Article DOI: 10.1016/j.ejmech.2013.01.039 BindingDB Entry DOI: 10.7270/Q2C82BN6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Equus caballus (Horse)) | BDBM50430725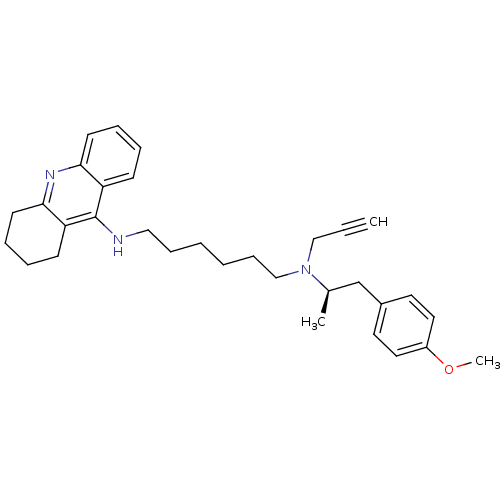 (CHEMBL2333931) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 29 | n/a | n/a | n/a | n/a | n/a | n/a |
Sun Yat-sen University Curated by ChEMBL | Assay Description Inhibition of horse serum BChE using butylthiocholine chloride as substrate after 15 mins by Ellman's method | Eur J Med Chem 62: 745-53 (2013) Article DOI: 10.1016/j.ejmech.2013.01.039 BindingDB Entry DOI: 10.7270/Q2C82BN6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Equus caballus (Horse)) | BDBM50430732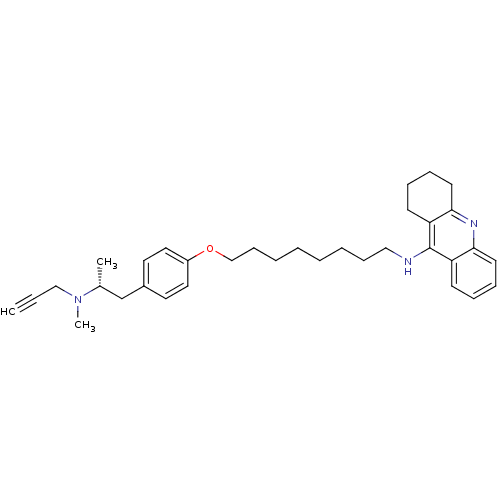 (CHEMBL2333937) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 36 | n/a | n/a | n/a | n/a | n/a | n/a |
Sun Yat-sen University Curated by ChEMBL | Assay Description Inhibition of horse serum BChE using butylthiocholine chloride as substrate after 15 mins by Ellman's method | Eur J Med Chem 62: 745-53 (2013) Article DOI: 10.1016/j.ejmech.2013.01.039 BindingDB Entry DOI: 10.7270/Q2C82BN6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM50430736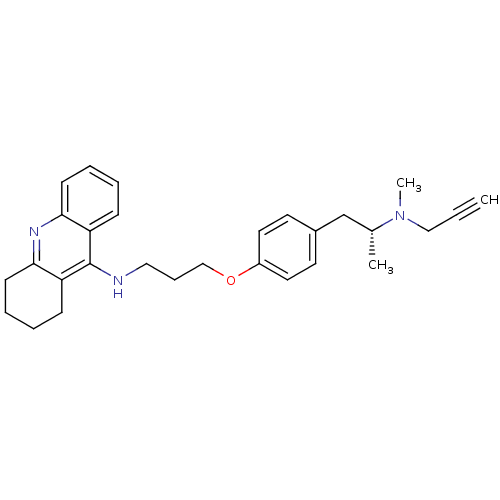 (CHEMBL2333933) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 36 | n/a | n/a | n/a | n/a | n/a | n/a |
Sun Yat-sen University Curated by ChEMBL | Assay Description Inhibition of electric eel AChE using acetylthiocholine chloride as substrate after 15 mins by Ellman's method | Eur J Med Chem 62: 745-53 (2013) Article DOI: 10.1016/j.ejmech.2013.01.039 BindingDB Entry DOI: 10.7270/Q2C82BN6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Equus caballus (Horse)) | BDBM50430735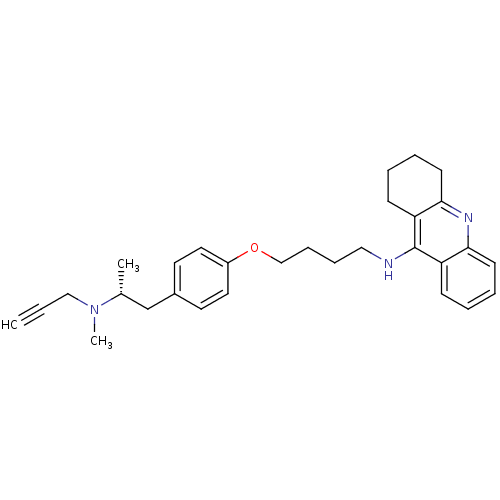 (CHEMBL2333934) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 40 | n/a | n/a | n/a | n/a | n/a | n/a |
Sun Yat-sen University Curated by ChEMBL | Assay Description Inhibition of horse serum BChE using butylthiocholine chloride as substrate after 15 mins by Ellman's method | Eur J Med Chem 62: 745-53 (2013) Article DOI: 10.1016/j.ejmech.2013.01.039 BindingDB Entry DOI: 10.7270/Q2C82BN6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Equus caballus (Horse)) | BDBM50430736 (CHEMBL2333933) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 41 | n/a | n/a | n/a | n/a | n/a | n/a |
Sun Yat-sen University Curated by ChEMBL | Assay Description Inhibition of horse serum BChE using butylthiocholine chloride as substrate after 15 mins by Ellman's method | Eur J Med Chem 62: 745-53 (2013) Article DOI: 10.1016/j.ejmech.2013.01.039 BindingDB Entry DOI: 10.7270/Q2C82BN6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Equus caballus (Horse)) | BDBM50430737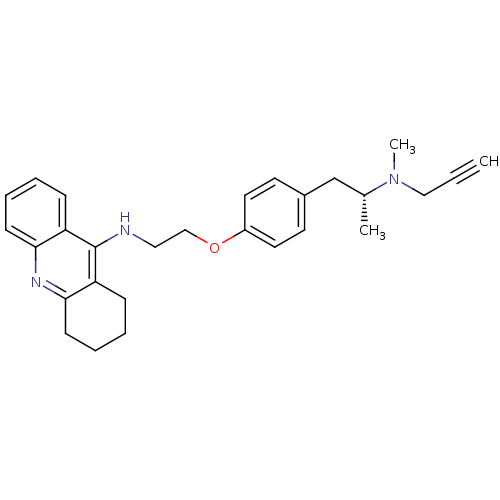 (CHEMBL2333932) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 51 | n/a | n/a | n/a | n/a | n/a | n/a |
Sun Yat-sen University Curated by ChEMBL | Assay Description Inhibition of horse serum BChE using butylthiocholine chloride as substrate after 15 mins by Ellman's method | Eur J Med Chem 62: 745-53 (2013) Article DOI: 10.1016/j.ejmech.2013.01.039 BindingDB Entry DOI: 10.7270/Q2C82BN6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM50430735 (CHEMBL2333934) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 53 | n/a | n/a | n/a | n/a | n/a | n/a |
Sun Yat-sen University Curated by ChEMBL | Assay Description Inhibition of electric eel AChE using acetylthiocholine chloride as substrate after 15 mins by Ellman's method | Eur J Med Chem 62: 745-53 (2013) Article DOI: 10.1016/j.ejmech.2013.01.039 BindingDB Entry DOI: 10.7270/Q2C82BN6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM50430726 (CHEMBL2333930) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 55 | n/a | n/a | n/a | n/a | n/a | n/a |
Sun Yat-sen University Curated by ChEMBL | Assay Description Inhibition of electric eel AChE using acetylthiocholine chloride as substrate after 15 mins by Ellman's method | Eur J Med Chem 62: 745-53 (2013) Article DOI: 10.1016/j.ejmech.2013.01.039 BindingDB Entry DOI: 10.7270/Q2C82BN6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Equus caballus (Horse)) | BDBM50430729 (CHEMBL2333927) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 66 | n/a | n/a | n/a | n/a | n/a | n/a |
Sun Yat-sen University Curated by ChEMBL | Assay Description Inhibition of horse serum BChE using butylthiocholine chloride as substrate after 15 mins by Ellman's method | Eur J Med Chem 62: 745-53 (2013) Article DOI: 10.1016/j.ejmech.2013.01.039 BindingDB Entry DOI: 10.7270/Q2C82BN6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM50430734 (CHEMBL2333935) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 78 | n/a | n/a | n/a | n/a | n/a | n/a |
Sun Yat-sen University Curated by ChEMBL | Assay Description Inhibition of electric eel AChE using acetylthiocholine chloride as substrate after 15 mins by Ellman's method | Eur J Med Chem 62: 745-53 (2013) Article DOI: 10.1016/j.ejmech.2013.01.039 BindingDB Entry DOI: 10.7270/Q2C82BN6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM50430727 (CHEMBL2333929) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 79 | n/a | n/a | n/a | n/a | n/a | n/a |
Sun Yat-sen University Curated by ChEMBL | Assay Description Inhibition of electric eel AChE using acetylthiocholine chloride as substrate after 15 mins by Ellman's method | Eur J Med Chem 62: 745-53 (2013) Article DOI: 10.1016/j.ejmech.2013.01.039 BindingDB Entry DOI: 10.7270/Q2C82BN6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM50430728 (CHEMBL2333928) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 82 | n/a | n/a | n/a | n/a | n/a | n/a |
Sun Yat-sen University Curated by ChEMBL | Assay Description Inhibition of electric eel AChE using acetylthiocholine chloride as substrate after 15 mins by Ellman's method | Eur J Med Chem 62: 745-53 (2013) Article DOI: 10.1016/j.ejmech.2013.01.039 BindingDB Entry DOI: 10.7270/Q2C82BN6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM50430737 (CHEMBL2333932) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 83 | n/a | n/a | n/a | n/a | n/a | n/a |
Sun Yat-sen University Curated by ChEMBL | Assay Description Inhibition of electric eel AChE using acetylthiocholine chloride as substrate after 15 mins by Ellman's method | Eur J Med Chem 62: 745-53 (2013) Article DOI: 10.1016/j.ejmech.2013.01.039 BindingDB Entry DOI: 10.7270/Q2C82BN6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM8961 (1,2,3,4-tetrahydro-9-acridinamine | 1,2,3,4-tetrah...) | UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | 110 | n/a | n/a | n/a | n/a | n/a | n/a |
Sun Yat-sen University Curated by ChEMBL | Assay Description Inhibition of electric eel AChE using acetylthiocholine chloride as substrate after 15 mins by Ellman's method | Eur J Med Chem 62: 745-53 (2013) Article DOI: 10.1016/j.ejmech.2013.01.039 BindingDB Entry DOI: 10.7270/Q2C82BN6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Amine oxidase [flavin-containing] B (Homo sapiens (Human)) | BDBM50430726 (CHEMBL2333930) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 129 | n/a | n/a | n/a | n/a | n/a | n/a |
Sun Yat-sen University Curated by ChEMBL | Assay Description Inhibition of recombinant human MAO-B using p-benzylamine substrate preincubated for 15 mins before substrate addition measured after 20 mins by fluo... | Eur J Med Chem 62: 745-53 (2013) Article DOI: 10.1016/j.ejmech.2013.01.039 BindingDB Entry DOI: 10.7270/Q2C82BN6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM50430733 (CHEMBL2333936) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 138 | n/a | n/a | n/a | n/a | n/a | n/a |
Sun Yat-sen University Curated by ChEMBL | Assay Description Inhibition of electric eel AChE using acetylthiocholine chloride as substrate after 15 mins by Ellman's method | Eur J Med Chem 62: 745-53 (2013) Article DOI: 10.1016/j.ejmech.2013.01.039 BindingDB Entry DOI: 10.7270/Q2C82BN6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM50430732 (CHEMBL2333937) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 147 | n/a | n/a | n/a | n/a | n/a | n/a |
Sun Yat-sen University Curated by ChEMBL | Assay Description Inhibition of electric eel AChE using acetylthiocholine chloride as substrate after 15 mins by Ellman's method | Eur J Med Chem 62: 745-53 (2013) Article DOI: 10.1016/j.ejmech.2013.01.039 BindingDB Entry DOI: 10.7270/Q2C82BN6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Amine oxidase [flavin-containing] B (Homo sapiens (Human)) | BDBM50430732 (CHEMBL2333937) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 167 | n/a | n/a | n/a | n/a | n/a | n/a |
Sun Yat-sen University Curated by ChEMBL | Assay Description Inhibition of recombinant human MAO-B using p-benzylamine substrate preincubated for 15 mins before substrate addition measured after 20 mins by fluo... | Eur J Med Chem 62: 745-53 (2013) Article DOI: 10.1016/j.ejmech.2013.01.039 BindingDB Entry DOI: 10.7270/Q2C82BN6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Amine oxidase [flavin-containing] B (Homo sapiens (Human)) | BDBM50430731 (CHEMBL2333925) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 181 | n/a | n/a | n/a | n/a | n/a | n/a |
Sun Yat-sen University Curated by ChEMBL | Assay Description Inhibition of recombinant human MAO-B using p-benzylamine substrate preincubated for 15 mins before substrate addition measured after 20 mins by fluo... | Eur J Med Chem 62: 745-53 (2013) Article DOI: 10.1016/j.ejmech.2013.01.039 BindingDB Entry DOI: 10.7270/Q2C82BN6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Amine oxidase [flavin-containing] B (Homo sapiens (Human)) | BDBM50172756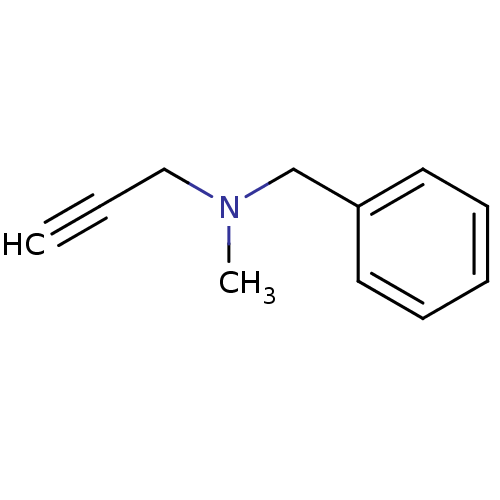 (Benzyl-methyl-prop-2-ynyl-amine | CHEMBL673 | Euto...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG PC cid PC sid UniChem Patents | Article PubMed | n/a | n/a | 188 | n/a | n/a | n/a | n/a | n/a | n/a |
Sun Yat-sen University Curated by ChEMBL | Assay Description Inhibition of recombinant human MAO-B using p-benzylamine substrate preincubated for 15 mins before substrate addition measured after 20 mins by fluo... | Eur J Med Chem 62: 745-53 (2013) Article DOI: 10.1016/j.ejmech.2013.01.039 BindingDB Entry DOI: 10.7270/Q2C82BN6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Amine oxidase [flavin-containing] A (Homo sapiens (Human)) | BDBM50430726 (CHEMBL2333930) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 193 | n/a | n/a | n/a | n/a | n/a | n/a |
Sun Yat-sen University Curated by ChEMBL | Assay Description Inhibition of recombinant human MAO-A using p-tyramine substrate preincubated for 15 mins before substrate addition measured after 20 mins by fluores... | Eur J Med Chem 62: 745-53 (2013) Article DOI: 10.1016/j.ejmech.2013.01.039 BindingDB Entry DOI: 10.7270/Q2C82BN6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Amine oxidase [flavin-containing] B (Homo sapiens (Human)) | BDBM50430733 (CHEMBL2333936) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 209 | n/a | n/a | n/a | n/a | n/a | n/a |
Sun Yat-sen University Curated by ChEMBL | Assay Description Inhibition of recombinant human MAO-B using p-benzylamine substrate preincubated for 15 mins before substrate addition measured after 20 mins by fluo... | Eur J Med Chem 62: 745-53 (2013) Article DOI: 10.1016/j.ejmech.2013.01.039 BindingDB Entry DOI: 10.7270/Q2C82BN6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Amine oxidase [flavin-containing] A (Homo sapiens (Human)) | BDBM50430730 (CHEMBL2333926) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 313 | n/a | n/a | n/a | n/a | n/a | n/a |
Sun Yat-sen University Curated by ChEMBL | Assay Description Inhibition of recombinant human MAO-A using p-tyramine substrate preincubated for 15 mins before substrate addition measured after 20 mins by fluores... | Eur J Med Chem 62: 745-53 (2013) Article DOI: 10.1016/j.ejmech.2013.01.039 BindingDB Entry DOI: 10.7270/Q2C82BN6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Amine oxidase [flavin-containing] B (Homo sapiens (Human)) | BDBM50430730 (CHEMBL2333926) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 346 | n/a | n/a | n/a | n/a | n/a | n/a |
Sun Yat-sen University Curated by ChEMBL | Assay Description Inhibition of recombinant human MAO-B using p-benzylamine substrate preincubated for 15 mins before substrate addition measured after 20 mins by fluo... | Eur J Med Chem 62: 745-53 (2013) Article DOI: 10.1016/j.ejmech.2013.01.039 BindingDB Entry DOI: 10.7270/Q2C82BN6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Amine oxidase [flavin-containing] A (Homo sapiens (Human)) | BDBM50430731 (CHEMBL2333925) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 372 | n/a | n/a | n/a | n/a | n/a | n/a |
Sun Yat-sen University Curated by ChEMBL | Assay Description Inhibition of recombinant human MAO-A using p-tyramine substrate preincubated for 15 mins before substrate addition measured after 20 mins by fluores... | Eur J Med Chem 62: 745-53 (2013) Article DOI: 10.1016/j.ejmech.2013.01.039 BindingDB Entry DOI: 10.7270/Q2C82BN6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Amine oxidase [flavin-containing] A (Homo sapiens (Human)) | BDBM50430733 (CHEMBL2333936) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 398 | n/a | n/a | n/a | n/a | n/a | n/a |
Sun Yat-sen University Curated by ChEMBL | Assay Description Inhibition of recombinant human MAO-A using p-tyramine substrate preincubated for 15 mins before substrate addition measured after 20 mins by fluores... | Eur J Med Chem 62: 745-53 (2013) Article DOI: 10.1016/j.ejmech.2013.01.039 BindingDB Entry DOI: 10.7270/Q2C82BN6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM50430725 (CHEMBL2333931) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 456 | n/a | n/a | n/a | n/a | n/a | n/a |
Sun Yat-sen University Curated by ChEMBL | Assay Description Inhibition of electric eel AChE using acetylthiocholine chloride as substrate after 15 mins by Ellman's method | Eur J Med Chem 62: 745-53 (2013) Article DOI: 10.1016/j.ejmech.2013.01.039 BindingDB Entry DOI: 10.7270/Q2C82BN6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Amine oxidase [flavin-containing] B (Homo sapiens (Human)) | BDBM50430727 (CHEMBL2333929) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 519 | n/a | n/a | n/a | n/a | n/a | n/a |
Sun Yat-sen University Curated by ChEMBL | Assay Description Inhibition of recombinant human MAO-B using p-benzylamine substrate preincubated for 15 mins before substrate addition measured after 20 mins by fluo... | Eur J Med Chem 62: 745-53 (2013) Article DOI: 10.1016/j.ejmech.2013.01.039 BindingDB Entry DOI: 10.7270/Q2C82BN6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Amine oxidase [flavin-containing] A (Homo sapiens (Human)) | BDBM50430727 (CHEMBL2333929) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 521 | n/a | n/a | n/a | n/a | n/a | n/a |
Sun Yat-sen University Curated by ChEMBL | Assay Description Inhibition of recombinant human MAO-A using p-tyramine substrate preincubated for 15 mins before substrate addition measured after 20 mins by fluores... | Eur J Med Chem 62: 745-53 (2013) Article DOI: 10.1016/j.ejmech.2013.01.039 BindingDB Entry DOI: 10.7270/Q2C82BN6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Amine oxidase [flavin-containing] A (Homo sapiens (Human)) | BDBM50430732 (CHEMBL2333937) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 591 | n/a | n/a | n/a | n/a | n/a | n/a |
Sun Yat-sen University Curated by ChEMBL | Assay Description Inhibition of recombinant human MAO-A using p-tyramine substrate preincubated for 15 mins before substrate addition measured after 20 mins by fluores... | Eur J Med Chem 62: 745-53 (2013) Article DOI: 10.1016/j.ejmech.2013.01.039 BindingDB Entry DOI: 10.7270/Q2C82BN6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Amine oxidase [flavin-containing] B (Homo sapiens (Human)) | BDBM50430737 (CHEMBL2333932) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 693 | n/a | n/a | n/a | n/a | n/a | n/a |
Sun Yat-sen University Curated by ChEMBL | Assay Description Inhibition of recombinant human MAO-B using p-benzylamine substrate preincubated for 15 mins before substrate addition measured after 20 mins by fluo... | Eur J Med Chem 62: 745-53 (2013) Article DOI: 10.1016/j.ejmech.2013.01.039 BindingDB Entry DOI: 10.7270/Q2C82BN6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Amine oxidase [flavin-containing] A (Homo sapiens (Human)) | BDBM50430734 (CHEMBL2333935) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.26E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Sun Yat-sen University Curated by ChEMBL | Assay Description Inhibition of recombinant human MAO-A using p-tyramine substrate preincubated for 15 mins before substrate addition measured after 20 mins by fluores... | Eur J Med Chem 62: 745-53 (2013) Article DOI: 10.1016/j.ejmech.2013.01.039 BindingDB Entry DOI: 10.7270/Q2C82BN6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Amine oxidase [flavin-containing] B (Homo sapiens (Human)) | BDBM50430734 (CHEMBL2333935) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2.40E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Sun Yat-sen University Curated by ChEMBL | Assay Description Inhibition of recombinant human MAO-B using p-benzylamine substrate preincubated for 15 mins before substrate addition measured after 20 mins by fluo... | Eur J Med Chem 62: 745-53 (2013) Article DOI: 10.1016/j.ejmech.2013.01.039 BindingDB Entry DOI: 10.7270/Q2C82BN6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Amine oxidase [flavin-containing] A (Homo sapiens (Human)) | BDBM50430728 (CHEMBL2333928) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2.86E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Sun Yat-sen University Curated by ChEMBL | Assay Description Inhibition of recombinant human MAO-A using p-tyramine substrate preincubated for 15 mins before substrate addition measured after 20 mins by fluores... | Eur J Med Chem 62: 745-53 (2013) Article DOI: 10.1016/j.ejmech.2013.01.039 BindingDB Entry DOI: 10.7270/Q2C82BN6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Amine oxidase [flavin-containing] B (Homo sapiens (Human)) | BDBM50430736 (CHEMBL2333933) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 4.46E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Sun Yat-sen University Curated by ChEMBL | Assay Description Inhibition of recombinant human MAO-B using p-benzylamine substrate preincubated for 15 mins before substrate addition measured after 20 mins by fluo... | Eur J Med Chem 62: 745-53 (2013) Article DOI: 10.1016/j.ejmech.2013.01.039 BindingDB Entry DOI: 10.7270/Q2C82BN6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Amine oxidase [flavin-containing] A (Homo sapiens (Human)) | BDBM50430737 (CHEMBL2333932) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 4.86E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Sun Yat-sen University Curated by ChEMBL | Assay Description Inhibition of recombinant human MAO-A using p-tyramine substrate preincubated for 15 mins before substrate addition measured after 20 mins by fluores... | Eur J Med Chem 62: 745-53 (2013) Article DOI: 10.1016/j.ejmech.2013.01.039 BindingDB Entry DOI: 10.7270/Q2C82BN6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Amine oxidase [flavin-containing] B (Homo sapiens (Human)) | BDBM50430728 (CHEMBL2333928) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 6.21E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Sun Yat-sen University Curated by ChEMBL | Assay Description Inhibition of recombinant human MAO-B using p-benzylamine substrate preincubated for 15 mins before substrate addition measured after 20 mins by fluo... | Eur J Med Chem 62: 745-53 (2013) Article DOI: 10.1016/j.ejmech.2013.01.039 BindingDB Entry DOI: 10.7270/Q2C82BN6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Amine oxidase [flavin-containing] A (Homo sapiens (Human)) | BDBM50430735 (CHEMBL2333934) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 7.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Sun Yat-sen University Curated by ChEMBL | Assay Description Inhibition of recombinant human MAO-A using p-tyramine substrate preincubated for 15 mins before substrate addition measured after 20 mins by fluores... | Eur J Med Chem 62: 745-53 (2013) Article DOI: 10.1016/j.ejmech.2013.01.039 BindingDB Entry DOI: 10.7270/Q2C82BN6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 56 total ) | Next | Last >> |