

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Amine oxidase [flavin-containing] A (Homo sapiens (Human)) | BDBM50467507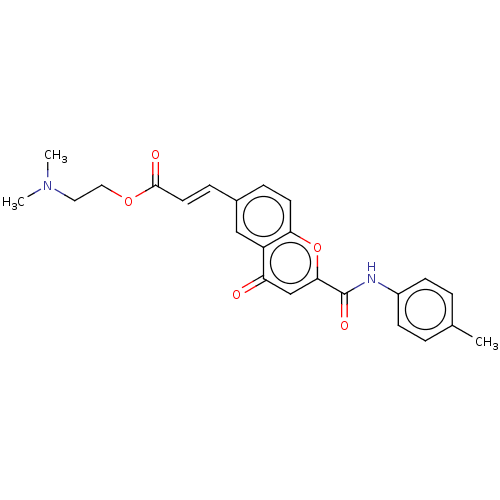 (CHEMBL4292235) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 57 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Porto Curated by ChEMBL | Assay Description Reversible competitive inhibition of human recombinant microsomal MAOA expressed in baculovirus infected BTI-TN-5B1- 4 cells using p-tyramine as subs... | Eur J Med Chem 158: 781-800 (2018) Article DOI: 10.1016/j.ejmech.2018.07.056 BindingDB Entry DOI: 10.7270/Q2F192DM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM50467507 (CHEMBL4292235) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 190 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Porto Curated by ChEMBL | Assay Description Mixed type inhibition of human AChE using acetylthiocholine iodide as substrate pretreated for 5 mins followed by substrate addition measured after 5... | Eur J Med Chem 158: 781-800 (2018) Article DOI: 10.1016/j.ejmech.2018.07.056 BindingDB Entry DOI: 10.7270/Q2F192DM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Amine oxidase [flavin-containing] B (Homo sapiens (Human)) | BDBM50467518 (CHEMBL4286556) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 337 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Porto Curated by ChEMBL | Assay Description Reversible competitive inhibition of human recombinant microsomal MAOB expressed in baculovirus infected BTI-TN-5B1- 4 cells using p-tyramine as subs... | Eur J Med Chem 158: 781-800 (2018) Article DOI: 10.1016/j.ejmech.2018.07.056 BindingDB Entry DOI: 10.7270/Q2F192DM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Amine oxidase [flavin-containing] B (Homo sapiens (Human)) | BDBM50467507 (CHEMBL4292235) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 478 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Porto Curated by ChEMBL | Assay Description Reversible competitive inhibition of human recombinant microsomal MAOB expressed in baculovirus infected BTI-TN-5B1- 4 cells using p-tyramine as subs... | Eur J Med Chem 158: 781-800 (2018) Article DOI: 10.1016/j.ejmech.2018.07.056 BindingDB Entry DOI: 10.7270/Q2F192DM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Amine oxidase [flavin-containing] A (Homo sapiens (Human)) | BDBM15581 (CHEMBL8706 | CLG | CLORGILINE | Clorgyline | N-[3-...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | n/a | n/a | 4.5 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Porto Curated by ChEMBL | Assay Description Inhibition of human recombinant microsomal MAOA expressed in baculovirus infected BTI-TN-5B1- 4 cells using p-tyramine as substrate assessed as decre... | Eur J Med Chem 158: 781-800 (2018) Article DOI: 10.1016/j.ejmech.2018.07.056 BindingDB Entry DOI: 10.7270/Q2F192DM | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM8960 ((+/-)-2-[(1-benzylpiperidin-4-yl)methyl]-5,6-dimet...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE PC cid PC sid PDB UniChem Patents Similars | DrugBank PDB Article PubMed | n/a | n/a | 10 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Porto Curated by ChEMBL | Assay Description Inhibition of human AChE using acetylthiocholine iodide as substrate pretreated for 5 mins followed by substrate addition measured after 5 mins by El... | Eur J Med Chem 158: 781-800 (2018) Article DOI: 10.1016/j.ejmech.2018.07.056 BindingDB Entry DOI: 10.7270/Q2F192DM | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Amine oxidase [flavin-containing] B (Homo sapiens (Human)) | BDBM15579 (CHEMBL972 | DEPRENYL | L-Deprenyl | N-methyl-N-[(2...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE KEGG PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 17 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Porto Curated by ChEMBL | Assay Description Inhibition of human recombinant MAOB expressed in baculovirus infected BTI-TN-5B1- 4 cells using p-tyramine as substrate assessed as decrease in H2O2... | Eur J Med Chem 158: 781-800 (2018) Article DOI: 10.1016/j.ejmech.2018.07.056 BindingDB Entry DOI: 10.7270/Q2F192DM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Amine oxidase [flavin-containing] B (Homo sapiens (Human)) | BDBM19187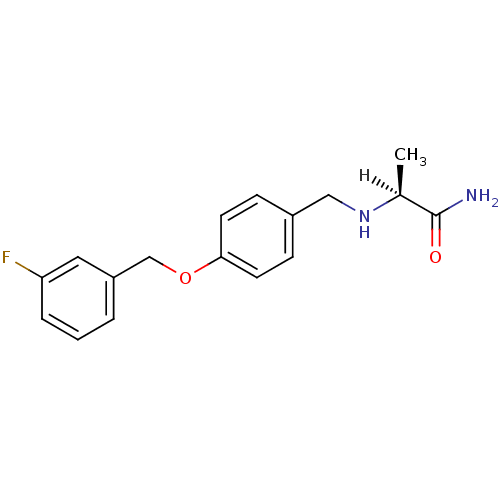 ((2S)-2-[({4-[(3-fluorophenyl)methoxy]phenyl}methyl...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE MMDB PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | n/a | n/a | 23 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Porto Curated by ChEMBL | Assay Description Inhibition of human recombinant MAOB expressed in baculovirus infected BTI-TN-5B1- 4 cells using p-tyramine as substrate assessed as decrease in H2O2... | Eur J Med Chem 158: 781-800 (2018) Article DOI: 10.1016/j.ejmech.2018.07.056 BindingDB Entry DOI: 10.7270/Q2F192DM | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Cholinesterase (Homo sapiens (Human)) | BDBM8961 (1,2,3,4-tetrahydro-9-acridinamine | 1,2,3,4-tetrah...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | n/a | n/a | 40 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Porto Curated by ChEMBL | Assay Description Inhibition of human serum BuChE using butyrylthiocholine as substrate pretreated for 5 mins followed by substrate addition measured after 5 mins by E... | Eur J Med Chem 158: 781-800 (2018) Article DOI: 10.1016/j.ejmech.2018.07.056 BindingDB Entry DOI: 10.7270/Q2F192DM | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Amine oxidase [flavin-containing] B (Homo sapiens (Human)) | BDBM10989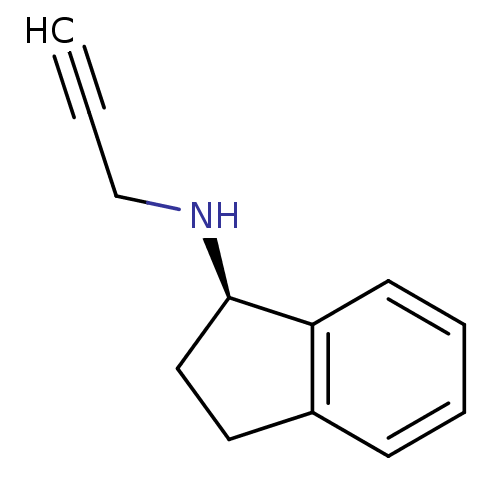 ((1R)-N-(prop-2-yn-1-yl)-2,3-dihydro-1H-inden-1-ami...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | 50 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Porto Curated by ChEMBL | Assay Description Inhibition of human recombinant MAOB expressed in baculovirus infected BTI-TN-5B1- 4 cells using p-tyramine as substrate assessed as decrease in H2O2... | Eur J Med Chem 158: 781-800 (2018) Article DOI: 10.1016/j.ejmech.2018.07.056 BindingDB Entry DOI: 10.7270/Q2F192DM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM50467530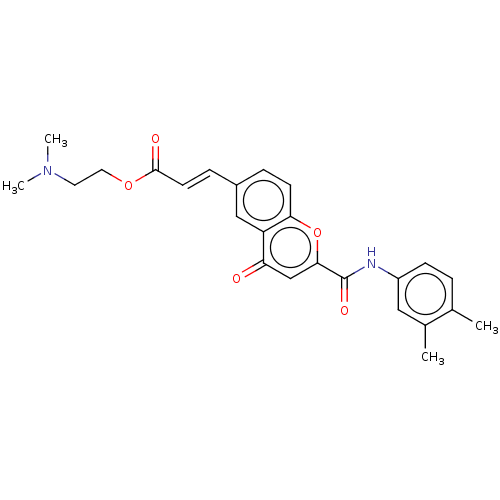 (CHEMBL4279297) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 210 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Porto Curated by ChEMBL | Assay Description Inhibition of human AChE using acetylthiocholine iodide as substrate pretreated for 5 mins followed by substrate addition measured after 5 mins by El... | Eur J Med Chem 158: 781-800 (2018) Article DOI: 10.1016/j.ejmech.2018.07.056 BindingDB Entry DOI: 10.7270/Q2F192DM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM50467507 (CHEMBL4292235) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 210 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Porto Curated by ChEMBL | Assay Description Inhibition of human AChE using acetylthiocholine iodide as substrate pretreated for 5 mins followed by substrate addition measured after 5 mins by El... | Eur J Med Chem 158: 781-800 (2018) Article DOI: 10.1016/j.ejmech.2018.07.056 BindingDB Entry DOI: 10.7270/Q2F192DM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM8961 (1,2,3,4-tetrahydro-9-acridinamine | 1,2,3,4-tetrah...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | n/a | n/a | 230 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Porto Curated by ChEMBL | Assay Description Inhibition of human AChE using acetylthiocholine iodide as substrate pretreated for 5 mins followed by substrate addition measured after 5 mins by El... | Eur J Med Chem 158: 781-800 (2018) Article DOI: 10.1016/j.ejmech.2018.07.056 BindingDB Entry DOI: 10.7270/Q2F192DM | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Amine oxidase [flavin-containing] B (Homo sapiens (Human)) | BDBM50467518 (CHEMBL4286556) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 630 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Porto Curated by ChEMBL | Assay Description Inhibition of human recombinant MAOB expressed in baculovirus infected BTI-TN-5B1- 4 cells using p-tyramine as substrate assessed as decrease in H2O2... | Eur J Med Chem 158: 781-800 (2018) Article DOI: 10.1016/j.ejmech.2018.07.056 BindingDB Entry DOI: 10.7270/Q2F192DM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Amine oxidase [flavin-containing] B (Homo sapiens (Human)) | BDBM50467514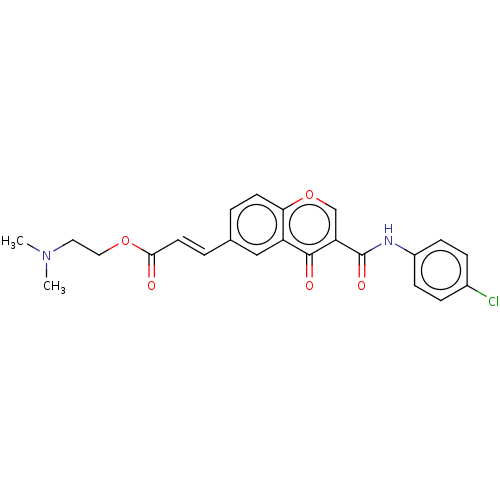 (CHEMBL4285749) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 720 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Porto Curated by ChEMBL | Assay Description Inhibition of human recombinant MAOB expressed in baculovirus infected BTI-TN-5B1- 4 cells using p-tyramine as substrate assessed as decrease in H2O2... | Eur J Med Chem 158: 781-800 (2018) Article DOI: 10.1016/j.ejmech.2018.07.056 BindingDB Entry DOI: 10.7270/Q2F192DM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM50467511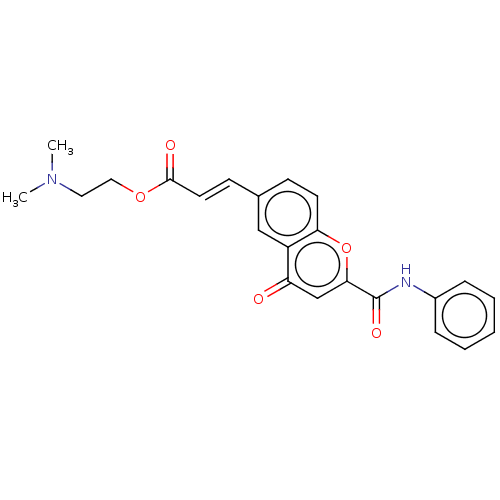 (CHEMBL4285714) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 740 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Porto Curated by ChEMBL | Assay Description Inhibition of human AChE using acetylthiocholine iodide as substrate pretreated for 5 mins followed by substrate addition measured after 5 mins by El... | Eur J Med Chem 158: 781-800 (2018) Article DOI: 10.1016/j.ejmech.2018.07.056 BindingDB Entry DOI: 10.7270/Q2F192DM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM50467515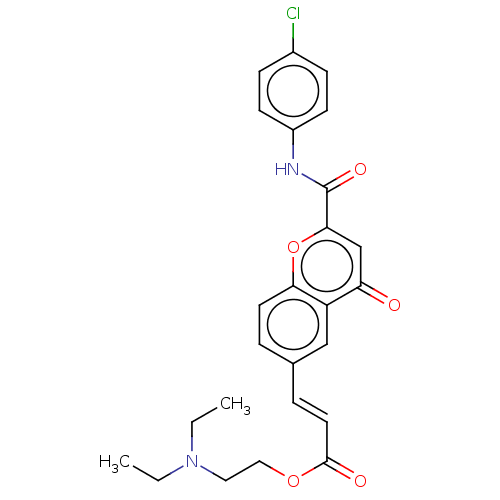 (CHEMBL4291046) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 860 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Porto Curated by ChEMBL | Assay Description Inhibition of human AChE using acetylthiocholine iodide as substrate pretreated for 5 mins followed by substrate addition measured after 5 mins by El... | Eur J Med Chem 158: 781-800 (2018) Article DOI: 10.1016/j.ejmech.2018.07.056 BindingDB Entry DOI: 10.7270/Q2F192DM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM50467519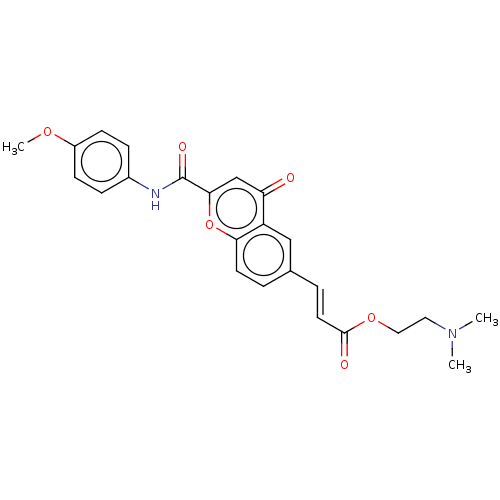 (CHEMBL4287595) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 910 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Porto Curated by ChEMBL | Assay Description Inhibition of human AChE using acetylthiocholine iodide as substrate pretreated for 5 mins followed by substrate addition measured after 5 mins by El... | Eur J Med Chem 158: 781-800 (2018) Article DOI: 10.1016/j.ejmech.2018.07.056 BindingDB Entry DOI: 10.7270/Q2F192DM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Amine oxidase [flavin-containing] A (Homo sapiens (Human)) | BDBM50467507 (CHEMBL4292235) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 940 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Porto Curated by ChEMBL | Assay Description Inhibition of human recombinant microsomal MAOA expressed in baculovirus infected BTI-TN-5B1- 4 cells using p-tyramine as substrate assessed as decre... | Eur J Med Chem 158: 781-800 (2018) Article DOI: 10.1016/j.ejmech.2018.07.056 BindingDB Entry DOI: 10.7270/Q2F192DM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM50467509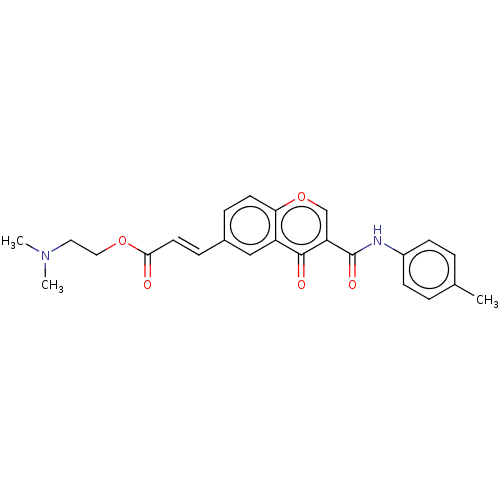 (CHEMBL4284235) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 970 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Porto Curated by ChEMBL | Assay Description Inhibition of human AChE using acetylthiocholine iodide as substrate pretreated for 5 mins followed by substrate addition measured after 5 mins by El... | Eur J Med Chem 158: 781-800 (2018) Article DOI: 10.1016/j.ejmech.2018.07.056 BindingDB Entry DOI: 10.7270/Q2F192DM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM50467528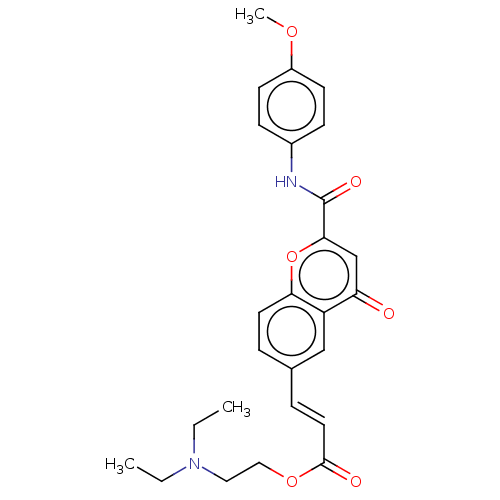 (CHEMBL4293364) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 1.14E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Porto Curated by ChEMBL | Assay Description Inhibition of human AChE using acetylthiocholine iodide as substrate pretreated for 5 mins followed by substrate addition measured after 5 mins by El... | Eur J Med Chem 158: 781-800 (2018) Article DOI: 10.1016/j.ejmech.2018.07.056 BindingDB Entry DOI: 10.7270/Q2F192DM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM50467510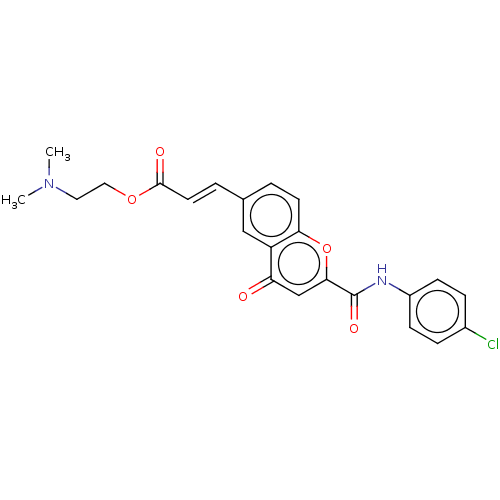 (CHEMBL4282037) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 1.22E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Porto Curated by ChEMBL | Assay Description Inhibition of human AChE using acetylthiocholine iodide as substrate pretreated for 5 mins followed by substrate addition measured after 5 mins by El... | Eur J Med Chem 158: 781-800 (2018) Article DOI: 10.1016/j.ejmech.2018.07.056 BindingDB Entry DOI: 10.7270/Q2F192DM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Amine oxidase [flavin-containing] B (Homo sapiens (Human)) | BDBM50467513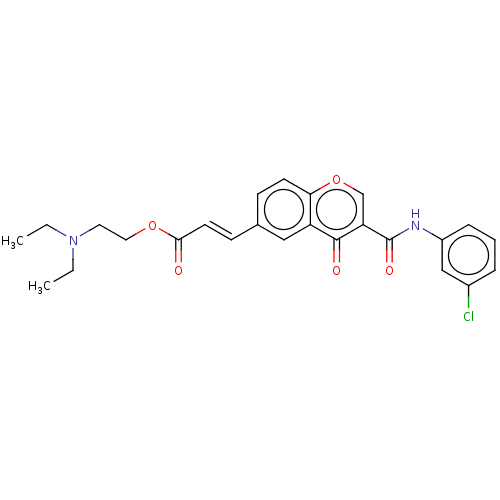 (CHEMBL4283463) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 1.25E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Porto Curated by ChEMBL | Assay Description Inhibition of human recombinant MAOB expressed in baculovirus infected BTI-TN-5B1- 4 cells using p-tyramine as substrate assessed as decrease in H2O2... | Eur J Med Chem 158: 781-800 (2018) Article DOI: 10.1016/j.ejmech.2018.07.056 BindingDB Entry DOI: 10.7270/Q2F192DM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Homo sapiens (Human)) | BDBM50467511 (CHEMBL4285714) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 1.57E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Porto Curated by ChEMBL | Assay Description Inhibition of human serum BuChE using butyrylthiocholine as substrate pretreated for 5 mins followed by substrate addition measured after 5 mins by E... | Eur J Med Chem 158: 781-800 (2018) Article DOI: 10.1016/j.ejmech.2018.07.056 BindingDB Entry DOI: 10.7270/Q2F192DM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Amine oxidase [flavin-containing] B (Homo sapiens (Human)) | BDBM50467532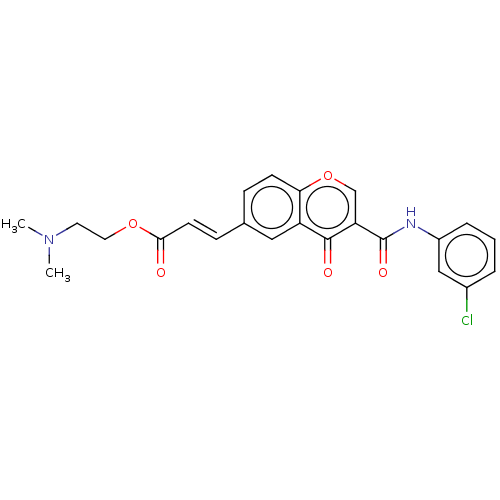 (CHEMBL4293612) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 2.21E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Porto Curated by ChEMBL | Assay Description Inhibition of human recombinant MAOB expressed in baculovirus infected BTI-TN-5B1- 4 cells using p-tyramine as substrate assessed as decrease in H2O2... | Eur J Med Chem 158: 781-800 (2018) Article DOI: 10.1016/j.ejmech.2018.07.056 BindingDB Entry DOI: 10.7270/Q2F192DM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Amine oxidase [flavin-containing] B (Homo sapiens (Human)) | BDBM50467511 (CHEMBL4285714) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 2.28E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Porto Curated by ChEMBL | Assay Description Inhibition of human recombinant MAOB expressed in baculovirus infected BTI-TN-5B1- 4 cells using p-tyramine as substrate assessed as decrease in H2O2... | Eur J Med Chem 158: 781-800 (2018) Article DOI: 10.1016/j.ejmech.2018.07.056 BindingDB Entry DOI: 10.7270/Q2F192DM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Amine oxidase [flavin-containing] B (Homo sapiens (Human)) | BDBM50467509 (CHEMBL4284235) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 2.37E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Porto Curated by ChEMBL | Assay Description Inhibition of human recombinant MAOB expressed in baculovirus infected BTI-TN-5B1- 4 cells using p-tyramine as substrate assessed as decrease in H2O2... | Eur J Med Chem 158: 781-800 (2018) Article DOI: 10.1016/j.ejmech.2018.07.056 BindingDB Entry DOI: 10.7270/Q2F192DM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM50467520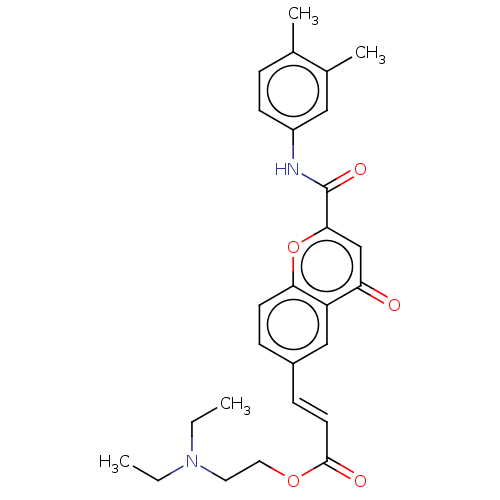 (CHEMBL4294446) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 2.47E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Porto Curated by ChEMBL | Assay Description Inhibition of human AChE using acetylthiocholine iodide as substrate pretreated for 5 mins followed by substrate addition measured after 5 mins by El... | Eur J Med Chem 158: 781-800 (2018) Article DOI: 10.1016/j.ejmech.2018.07.056 BindingDB Entry DOI: 10.7270/Q2F192DM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Homo sapiens (Human)) | BDBM8960 ((+/-)-2-[(1-benzylpiperidin-4-yl)methyl]-5,6-dimet...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | 2.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Porto Curated by ChEMBL | Assay Description Inhibition of human serum BuChE using butyrylthiocholine as substrate pretreated for 5 mins followed by substrate addition measured after 5 mins by E... | Eur J Med Chem 158: 781-800 (2018) Article DOI: 10.1016/j.ejmech.2018.07.056 BindingDB Entry DOI: 10.7270/Q2F192DM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Amine oxidase [flavin-containing] B (Homo sapiens (Human)) | BDBM50467520 (CHEMBL4294446) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 2.54E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Porto Curated by ChEMBL | Assay Description Inhibition of human recombinant MAOB expressed in baculovirus infected BTI-TN-5B1- 4 cells using p-tyramine as substrate assessed as decrease in H2O2... | Eur J Med Chem 158: 781-800 (2018) Article DOI: 10.1016/j.ejmech.2018.07.056 BindingDB Entry DOI: 10.7270/Q2F192DM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM50467529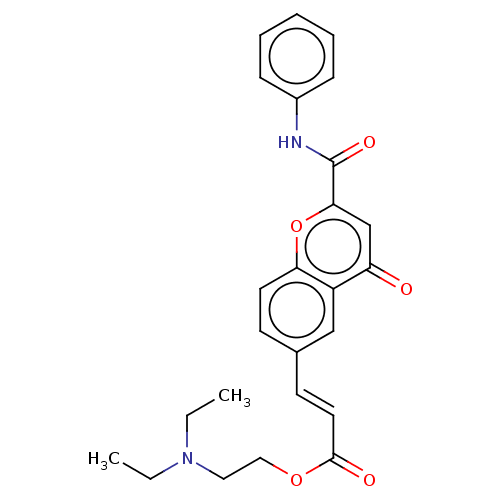 (CHEMBL4289981) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 3.47E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Porto Curated by ChEMBL | Assay Description Inhibition of human AChE using acetylthiocholine iodide as substrate pretreated for 5 mins followed by substrate addition measured after 5 mins by El... | Eur J Med Chem 158: 781-800 (2018) Article DOI: 10.1016/j.ejmech.2018.07.056 BindingDB Entry DOI: 10.7270/Q2F192DM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM50467533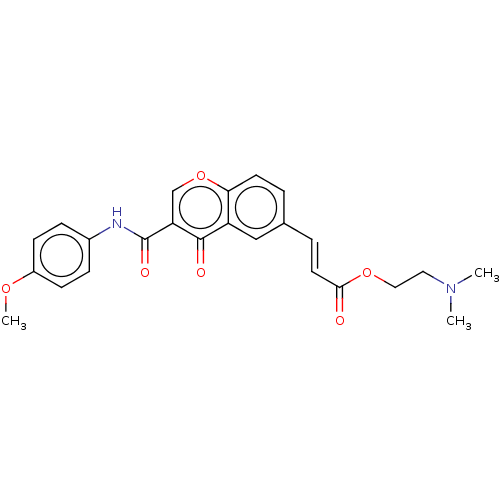 (CHEMBL4294859) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 3.54E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Porto Curated by ChEMBL | Assay Description Inhibition of human AChE using acetylthiocholine iodide as substrate pretreated for 5 mins followed by substrate addition measured after 5 mins by El... | Eur J Med Chem 158: 781-800 (2018) Article DOI: 10.1016/j.ejmech.2018.07.056 BindingDB Entry DOI: 10.7270/Q2F192DM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Amine oxidase [flavin-containing] B (Homo sapiens (Human)) | BDBM50467531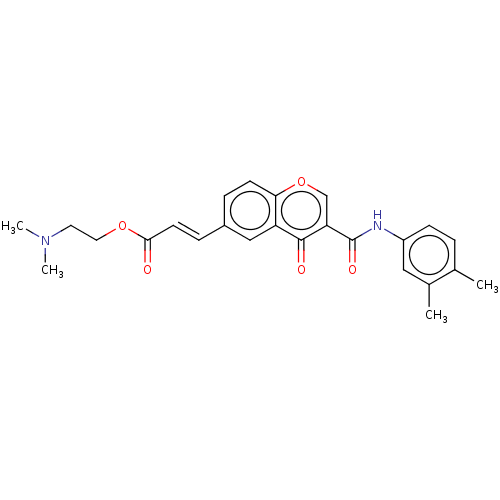 (CHEMBL4282350) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 3.65E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Porto Curated by ChEMBL | Assay Description Inhibition of human recombinant MAOB expressed in baculovirus infected BTI-TN-5B1- 4 cells using p-tyramine as substrate assessed as decrease in H2O2... | Eur J Med Chem 158: 781-800 (2018) Article DOI: 10.1016/j.ejmech.2018.07.056 BindingDB Entry DOI: 10.7270/Q2F192DM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM50467518 (CHEMBL4286556) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 3.69E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Porto Curated by ChEMBL | Assay Description Inhibition of human AChE using acetylthiocholine iodide as substrate pretreated for 5 mins followed by substrate addition measured after 5 mins by El... | Eur J Med Chem 158: 781-800 (2018) Article DOI: 10.1016/j.ejmech.2018.07.056 BindingDB Entry DOI: 10.7270/Q2F192DM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Amine oxidase [flavin-containing] B (Homo sapiens (Human)) | BDBM50467507 (CHEMBL4292235) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 3.81E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Porto Curated by ChEMBL | Assay Description Inhibition of human recombinant MAOB expressed in baculovirus infected BTI-TN-5B1- 4 cells using p-tyramine as substrate assessed as decrease in H2O2... | Eur J Med Chem 158: 781-800 (2018) Article DOI: 10.1016/j.ejmech.2018.07.056 BindingDB Entry DOI: 10.7270/Q2F192DM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Homo sapiens (Human)) | BDBM50467518 (CHEMBL4286556) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 4.32E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Porto Curated by ChEMBL | Assay Description Inhibition of human serum BuChE using butyrylthiocholine as substrate pretreated for 5 mins followed by substrate addition measured after 5 mins by E... | Eur J Med Chem 158: 781-800 (2018) Article DOI: 10.1016/j.ejmech.2018.07.056 BindingDB Entry DOI: 10.7270/Q2F192DM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM50467527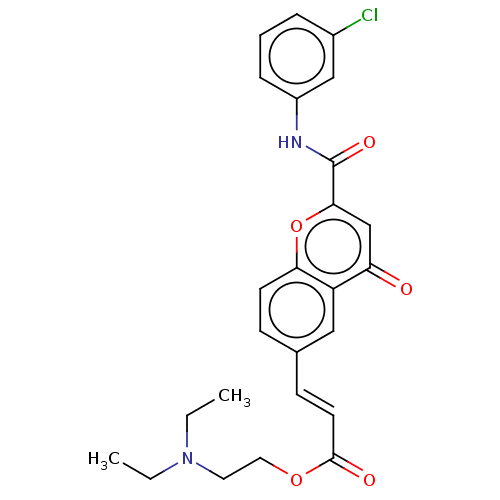 (CHEMBL4283189) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 4.45E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Porto Curated by ChEMBL | Assay Description Inhibition of human AChE using acetylthiocholine iodide as substrate pretreated for 5 mins followed by substrate addition measured after 5 mins by El... | Eur J Med Chem 158: 781-800 (2018) Article DOI: 10.1016/j.ejmech.2018.07.056 BindingDB Entry DOI: 10.7270/Q2F192DM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Amine oxidase [flavin-containing] B (Homo sapiens (Human)) | BDBM50467517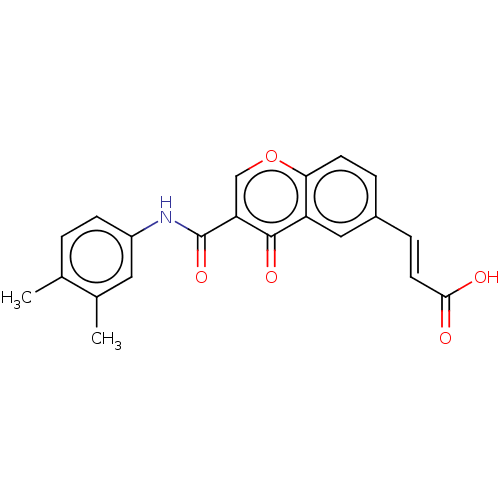 (CHEMBL4286877) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 4.52E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Porto Curated by ChEMBL | Assay Description Inhibition of human recombinant MAOB expressed in baculovirus infected BTI-TN-5B1- 4 cells using p-tyramine as substrate assessed as decrease in H2O2... | Eur J Med Chem 158: 781-800 (2018) Article DOI: 10.1016/j.ejmech.2018.07.056 BindingDB Entry DOI: 10.7270/Q2F192DM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Amine oxidase [flavin-containing] B (Homo sapiens (Human)) | BDBM50467508 (CHEMBL4284580) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 4.66E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Porto Curated by ChEMBL | Assay Description Inhibition of human recombinant MAOB expressed in baculovirus infected BTI-TN-5B1- 4 cells using p-tyramine as substrate assessed as decrease in H2O2... | Eur J Med Chem 158: 781-800 (2018) Article DOI: 10.1016/j.ejmech.2018.07.056 BindingDB Entry DOI: 10.7270/Q2F192DM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Amine oxidase [flavin-containing] A (Homo sapiens (Human)) | BDBM50467519 (CHEMBL4287595) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 4.91E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Porto Curated by ChEMBL | Assay Description Inhibition of human recombinant microsomal MAOA expressed in baculovirus infected BTI-TN-5B1- 4 cells using p-tyramine as substrate assessed as decre... | Eur J Med Chem 158: 781-800 (2018) Article DOI: 10.1016/j.ejmech.2018.07.056 BindingDB Entry DOI: 10.7270/Q2F192DM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM50467522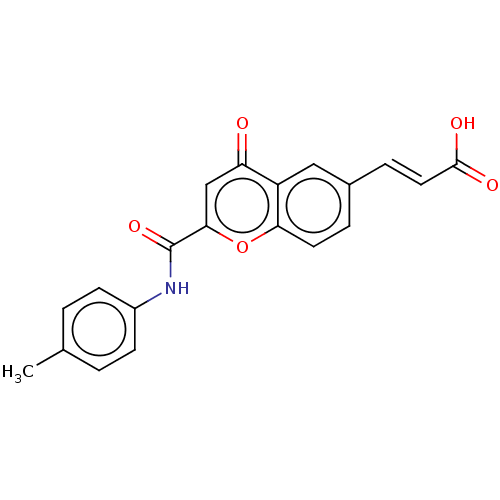 (CHEMBL4283814) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 5.10E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Porto Curated by ChEMBL | Assay Description Inhibition of human AChE using acetylthiocholine iodide as substrate pretreated for 5 mins followed by substrate addition measured after 5 mins by El... | Eur J Med Chem 158: 781-800 (2018) Article DOI: 10.1016/j.ejmech.2018.07.056 BindingDB Entry DOI: 10.7270/Q2F192DM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM50467525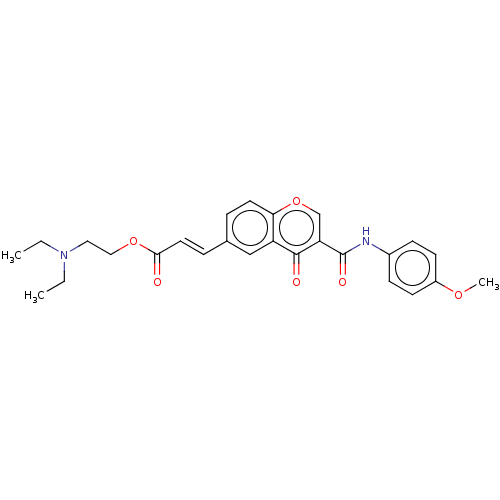 (CHEMBL4286806) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 5.43E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Porto Curated by ChEMBL | Assay Description Inhibition of human AChE using acetylthiocholine iodide as substrate pretreated for 5 mins followed by substrate addition measured after 5 mins by El... | Eur J Med Chem 158: 781-800 (2018) Article DOI: 10.1016/j.ejmech.2018.07.056 BindingDB Entry DOI: 10.7270/Q2F192DM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM50467516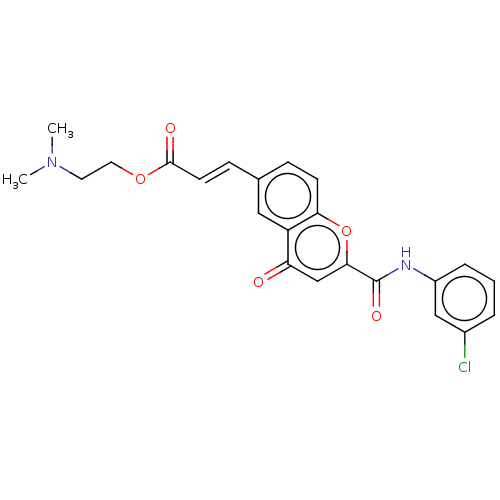 (CHEMBL4289908) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 5.92E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Porto Curated by ChEMBL | Assay Description Inhibition of human AChE using acetylthiocholine iodide as substrate pretreated for 5 mins followed by substrate addition measured after 5 mins by El... | Eur J Med Chem 158: 781-800 (2018) Article DOI: 10.1016/j.ejmech.2018.07.056 BindingDB Entry DOI: 10.7270/Q2F192DM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM50467523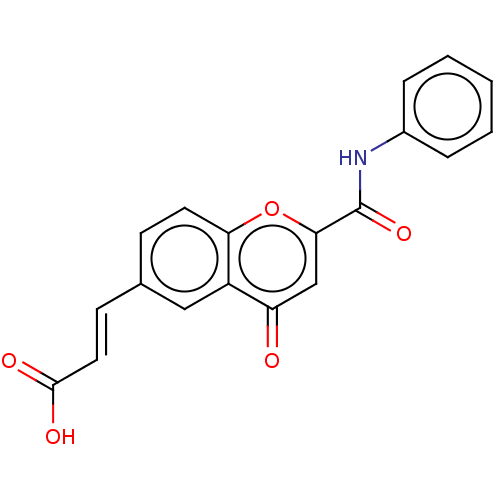 (CHEMBL4279249) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 5.99E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Porto Curated by ChEMBL | Assay Description Inhibition of human AChE using acetylthiocholine iodide as substrate pretreated for 5 mins followed by substrate addition measured after 5 mins by El... | Eur J Med Chem 158: 781-800 (2018) Article DOI: 10.1016/j.ejmech.2018.07.056 BindingDB Entry DOI: 10.7270/Q2F192DM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Homo sapiens (Human)) | BDBM50467509 (CHEMBL4284235) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 7.42E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Porto Curated by ChEMBL | Assay Description Inhibition of human serum BuChE using butyrylthiocholine as substrate pretreated for 5 mins followed by substrate addition measured after 5 mins by E... | Eur J Med Chem 158: 781-800 (2018) Article DOI: 10.1016/j.ejmech.2018.07.056 BindingDB Entry DOI: 10.7270/Q2F192DM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM50467521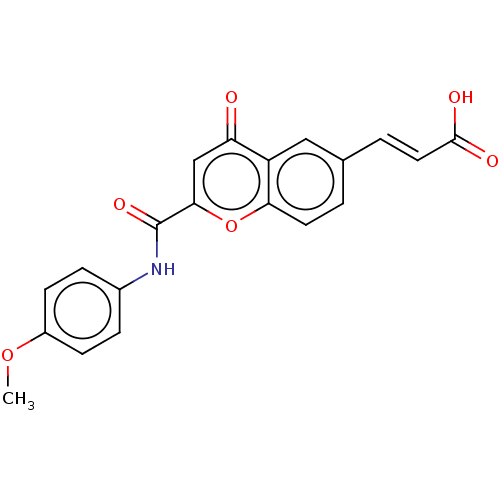 (CHEMBL4294389) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 7.43E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Porto Curated by ChEMBL | Assay Description Inhibition of human AChE using acetylthiocholine iodide as substrate pretreated for 5 mins followed by substrate addition measured after 5 mins by El... | Eur J Med Chem 158: 781-800 (2018) Article DOI: 10.1016/j.ejmech.2018.07.056 BindingDB Entry DOI: 10.7270/Q2F192DM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM50467524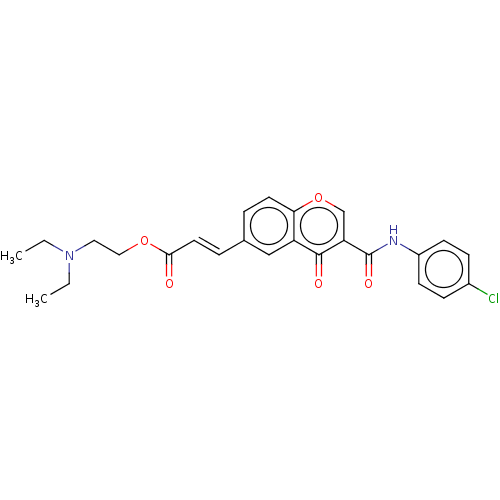 (CHEMBL4294028) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 7.68E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Porto Curated by ChEMBL | Assay Description Inhibition of human AChE using acetylthiocholine iodide as substrate pretreated for 5 mins followed by substrate addition measured after 5 mins by El... | Eur J Med Chem 158: 781-800 (2018) Article DOI: 10.1016/j.ejmech.2018.07.056 BindingDB Entry DOI: 10.7270/Q2F192DM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM50467513 (CHEMBL4283463) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 8.54E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Porto Curated by ChEMBL | Assay Description Inhibition of human AChE using acetylthiocholine iodide as substrate pretreated for 5 mins followed by substrate addition measured after 5 mins by El... | Eur J Med Chem 158: 781-800 (2018) Article DOI: 10.1016/j.ejmech.2018.07.056 BindingDB Entry DOI: 10.7270/Q2F192DM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM50467512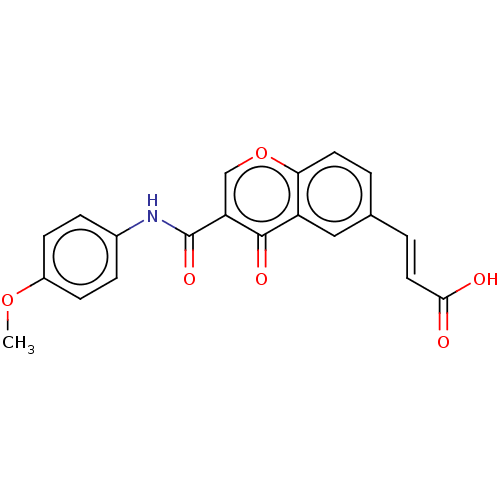 (CHEMBL4287962) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 8.70E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Porto Curated by ChEMBL | Assay Description Inhibition of human AChE using acetylthiocholine iodide as substrate pretreated for 5 mins followed by substrate addition measured after 5 mins by El... | Eur J Med Chem 158: 781-800 (2018) Article DOI: 10.1016/j.ejmech.2018.07.056 BindingDB Entry DOI: 10.7270/Q2F192DM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM50467526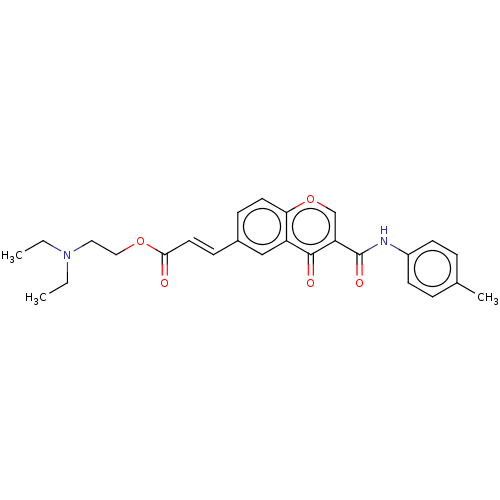 (CHEMBL4278883) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 8.99E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Porto Curated by ChEMBL | Assay Description Inhibition of human AChE using acetylthiocholine iodide as substrate pretreated for 5 mins followed by substrate addition measured after 5 mins by El... | Eur J Med Chem 158: 781-800 (2018) Article DOI: 10.1016/j.ejmech.2018.07.056 BindingDB Entry DOI: 10.7270/Q2F192DM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 61 total ) | Next | Last >> |