 Found 18 hits Enz. Inhib. hit(s) with all data for entry = 50003577
Found 18 hits Enz. Inhib. hit(s) with all data for entry = 50003577 Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Reverse transcriptase/RNaseH
(Human immunodeficiency virus 1) | BDBM2483

((4S)-6-chloro-4-(2-cyclopropylethynyl)-4-(trifluor...)Show SMILES FC(F)(F)[C@]1(OC(=O)Nc2ccc(Cl)cc12)C#CC1CC1 |r| Show InChI InChI=1S/C14H9ClF3NO2/c15-9-3-4-11-10(7-9)13(14(16,17)18,21-12(20)19-11)6-5-8-1-2-8/h3-4,7-8H,1-2H2,(H,19,20)/t13-/m0/s1 | PDB
MMDB
UniProtKB/TrEMBL
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| DrugBank
MMDB
PDB
Article
PubMed
| n/a | n/a | 9.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Universidad de Alcal£
Curated by ChEMBL
| Assay Description
Inhibitory activity against R172A mutant reverse transcriptase |
J Med Chem 44: 1853-65 (2001)
Article DOI: 10.1021/jm001095i
BindingDB Entry DOI: 10.7270/Q20V8GJ7 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Reverse transcriptase/RNaseH
(Human immunodeficiency virus 1) | BDBM50105629

(CHEMBL54893 | N-(4-chloro-3-(3-methylbut-2-enyloxy...)Show SMILES [#6]\[#6](-[#6])=[#6]/[#6]-[#8]-c1cc(-[#7]-[#6](=S)-c2ccoc2-[#6])ccc1Cl Show InChI InChI=1S/C17H18ClNO2S/c1-11(2)6-8-21-16-10-13(4-5-15(16)18)19-17(22)14-7-9-20-12(14)3/h4-7,9-10H,8H2,1-3H3,(H,19,22) | PDB
MMDB
UniProtKB/TrEMBL
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
| MMDB
PDB
Article
PubMed
| n/a | n/a | 20.8 | n/a | n/a | n/a | n/a | n/a | n/a |
Universidad de Alcal£
Curated by ChEMBL
| Assay Description
Inhibitory activity against R172A mutant reverse transcriptase |
J Med Chem 44: 1853-65 (2001)
Article DOI: 10.1021/jm001095i
BindingDB Entry DOI: 10.7270/Q20V8GJ7 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Reverse transcriptase/RNaseH
(Human immunodeficiency virus 1) | BDBM2483

((4S)-6-chloro-4-(2-cyclopropylethynyl)-4-(trifluor...)Show SMILES FC(F)(F)[C@]1(OC(=O)Nc2ccc(Cl)cc12)C#CC1CC1 |r| Show InChI InChI=1S/C14H9ClF3NO2/c15-9-3-4-11-10(7-9)13(14(16,17)18,21-12(20)19-11)6-5-8-1-2-8/h3-4,7-8H,1-2H2,(H,19,20)/t13-/m0/s1 | PDB
MMDB
UniProtKB/TrEMBL
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| DrugBank
MMDB
PDB
Article
PubMed
| n/a | n/a | 22.2 | n/a | n/a | n/a | n/a | n/a | n/a |
Universidad de Alcal£
Curated by ChEMBL
| Assay Description
Inhibitory activity against wild-type HIV-1 reverse transcriptase |
J Med Chem 44: 1853-65 (2001)
Article DOI: 10.1021/jm001095i
BindingDB Entry DOI: 10.7270/Q20V8GJ7 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Reverse transcriptase/RNaseH
(Human immunodeficiency virus 1) | BDBM2483

((4S)-6-chloro-4-(2-cyclopropylethynyl)-4-(trifluor...)Show SMILES FC(F)(F)[C@]1(OC(=O)Nc2ccc(Cl)cc12)C#CC1CC1 |r| Show InChI InChI=1S/C14H9ClF3NO2/c15-9-3-4-11-10(7-9)13(14(16,17)18,21-12(20)19-11)6-5-8-1-2-8/h3-4,7-8H,1-2H2,(H,19,20)/t13-/m0/s1 | PDB
MMDB
UniProtKB/TrEMBL
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| DrugBank
MMDB
PDB
Article
PubMed
| n/a | n/a | 22.2 | n/a | n/a | n/a | n/a | n/a | n/a |
Universidad de Alcal£
Curated by ChEMBL
| Assay Description
Inhibitory activity against E138K mutant reverse transcriptase |
J Med Chem 44: 1853-65 (2001)
Article DOI: 10.1021/jm001095i
BindingDB Entry DOI: 10.7270/Q20V8GJ7 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Reverse transcriptase/RNaseH
(Human immunodeficiency virus 1) | BDBM50105629

(CHEMBL54893 | N-(4-chloro-3-(3-methylbut-2-enyloxy...)Show SMILES [#6]\[#6](-[#6])=[#6]/[#6]-[#8]-c1cc(-[#7]-[#6](=S)-c2ccoc2-[#6])ccc1Cl Show InChI InChI=1S/C17H18ClNO2S/c1-11(2)6-8-21-16-10-13(4-5-15(16)18)19-17(22)14-7-9-20-12(14)3/h4-7,9-10H,8H2,1-3H3,(H,19,22) | PDB
MMDB
UniProtKB/TrEMBL
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
| MMDB
PDB
Article
PubMed
| n/a | n/a | 23.8 | n/a | n/a | n/a | n/a | n/a | n/a |
Universidad de Alcal£
Curated by ChEMBL
| Assay Description
Inhibitory activity against wild-type HIV-1 reverse transcriptase |
J Med Chem 44: 1853-65 (2001)
Article DOI: 10.1021/jm001095i
BindingDB Entry DOI: 10.7270/Q20V8GJ7 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Reverse transcriptase/RNaseH
(Human immunodeficiency virus 1) | BDBM50105629

(CHEMBL54893 | N-(4-chloro-3-(3-methylbut-2-enyloxy...)Show SMILES [#6]\[#6](-[#6])=[#6]/[#6]-[#8]-c1cc(-[#7]-[#6](=S)-c2ccoc2-[#6])ccc1Cl Show InChI InChI=1S/C17H18ClNO2S/c1-11(2)6-8-21-16-10-13(4-5-15(16)18)19-17(22)14-7-9-20-12(14)3/h4-7,9-10H,8H2,1-3H3,(H,19,22) | PDB
MMDB
UniProtKB/TrEMBL
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
| MMDB
PDB
Article
PubMed
| n/a | n/a | 226 | n/a | n/a | n/a | n/a | n/a | n/a |
Universidad de Alcal£
Curated by ChEMBL
| Assay Description
Inhibitory activity against E138K mutant reverse transcriptase |
J Med Chem 44: 1853-65 (2001)
Article DOI: 10.1021/jm001095i
BindingDB Entry DOI: 10.7270/Q20V8GJ7 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Reverse transcriptase/RNaseH
(Human immunodeficiency virus 1) | BDBM1944

(BHAP deriv. | CHEMBL593 | DELAVIRDINE MESYLATE | D...)Show SMILES CC(C)Nc1cccnc1N1CCN(CC1)C(=O)c1cc2cc(NS(C)(=O)=O)ccc2[nH]1 Show InChI InChI=1S/C22H28N6O3S/c1-15(2)24-19-5-4-8-23-21(19)27-9-11-28(12-10-27)22(29)20-14-16-13-17(26-32(3,30)31)6-7-18(16)25-20/h4-8,13-15,24-26H,9-12H2,1-3H3 | PDB
MMDB
UniProtKB/TrEMBL
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| DrugBank
MMDB
PDB
Article
PubMed
| n/a | n/a | 394 | n/a | n/a | n/a | n/a | n/a | n/a |
Universidad de Alcal£
Curated by ChEMBL
| Assay Description
Inhibitory activity against R172A mutant reverse transcriptase |
J Med Chem 44: 1853-65 (2001)
Article DOI: 10.1021/jm001095i
BindingDB Entry DOI: 10.7270/Q20V8GJ7 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Reverse transcriptase/RNaseH
(Human immunodeficiency virus 1) | BDBM50473243
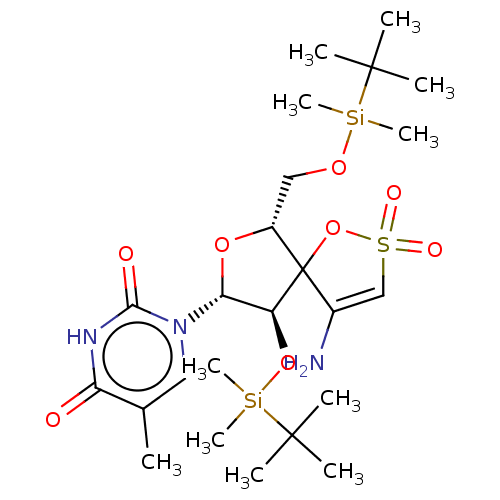
(CHEMBL2115411)Show SMILES Cc1cn([C@@H]2O[C@H](CO[Si](C)(C)C(C)(C)C)C3(OS(=O)(=O)C=C3N)[C@H]2O[Si](C)(C)C(C)(C)C)c(=O)[nH]c1=O |r,c:21| Show InChI InChI=1S/C24H43N3O8SSi2/c1-15-12-27(21(29)26-19(15)28)20-18(34-38(10,11)23(5,6)7)24(16(25)14-36(30,31)35-24)17(33-20)13-32-37(8,9)22(2,3)4/h12,14,17-18,20H,13,25H2,1-11H3,(H,26,28,29)/t17-,18+,20-,24?/m1/s1 | PDB
MMDB
UniProtKB/TrEMBL
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 627 | n/a | n/a | n/a | n/a | n/a | n/a |
Universidad de Alcal£
Curated by ChEMBL
| Assay Description
Inhibitory activity against R172A mutant reverse transcriptase |
J Med Chem 44: 1853-65 (2001)
Article DOI: 10.1021/jm001095i
BindingDB Entry DOI: 10.7270/Q20V8GJ7 |
More data for this
Ligand-Target Pair | |
Reverse transcriptase/RNaseH
(Human immunodeficiency virus 1) | BDBM1944

(BHAP deriv. | CHEMBL593 | DELAVIRDINE MESYLATE | D...)Show SMILES CC(C)Nc1cccnc1N1CCN(CC1)C(=O)c1cc2cc(NS(C)(=O)=O)ccc2[nH]1 Show InChI InChI=1S/C22H28N6O3S/c1-15(2)24-19-5-4-8-23-21(19)27-9-11-28(12-10-27)22(29)20-14-16-13-17(26-32(3,30)31)6-7-18(16)25-20/h4-8,13-15,24-26H,9-12H2,1-3H3 | PDB
MMDB
UniProtKB/TrEMBL
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| DrugBank
MMDB
PDB
Article
PubMed
| n/a | n/a | 657 | n/a | n/a | n/a | n/a | n/a | n/a |
Universidad de Alcal£
Curated by ChEMBL
| Assay Description
Inhibitory activity against wild-type HIV-1 reverse transcriptase |
J Med Chem 44: 1853-65 (2001)
Article DOI: 10.1021/jm001095i
BindingDB Entry DOI: 10.7270/Q20V8GJ7 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Reverse transcriptase/RNaseH
(Human immunodeficiency virus 1) | BDBM50192289
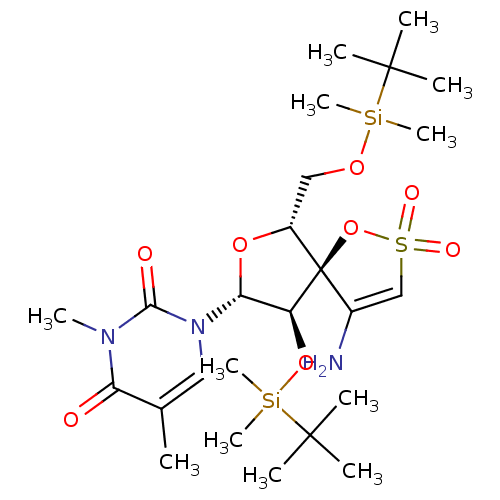
(1-[(5R,6R,8R,9R)-4-amino-9-(tert-butyl-dimethyl-si...)Show SMILES Cc1cn([C@@H]2O[C@H](CO[Si](C)(C)C(C)(C)C)[C@@]3(OS(=O)(=O)C=C3N)[C@H]2O[Si](C)(C)C(C)(C)C)c(=O)n(C)c1=O |c:21| Show InChI InChI=1S/C25H45N3O8SSi2/c1-16-13-28(22(30)27(8)20(16)29)21-19(35-39(11,12)24(5,6)7)25(17(26)15-37(31,32)36-25)18(34-21)14-33-38(9,10)23(2,3)4/h13,15,18-19,21H,14,26H2,1-12H3/t18-,19+,21-,25-/m1/s1 | PDB
MMDB
UniProtKB/TrEMBL
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 695 | n/a | n/a | n/a | n/a | n/a | n/a |
Universidad de Alcal£
Curated by ChEMBL
| Assay Description
Inhibitory activity against R172A mutant reverse transcriptase |
J Med Chem 44: 1853-65 (2001)
Article DOI: 10.1021/jm001095i
BindingDB Entry DOI: 10.7270/Q20V8GJ7 |
More data for this
Ligand-Target Pair | |
Reverse transcriptase/RNaseH
(Human immunodeficiency virus 1) | BDBM50369390
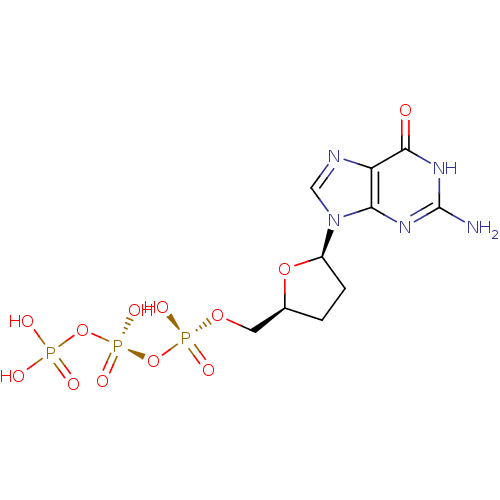
(CHEMBL54224)Show SMILES Nc1nc2n(cnc2c(=O)[nH]1)[C@H]1CC[C@@H](CO[P@](O)(=O)O[P@@](O)(=O)OP(O)(O)=O)O1 |r| Show InChI InChI=1S/C10H16N5O12P3/c11-10-13-8-7(9(16)14-10)12-4-15(8)6-2-1-5(25-6)3-24-29(20,21)27-30(22,23)26-28(17,18)19/h4-6H,1-3H2,(H,20,21)(H,22,23)(H2,17,18,19)(H3,11,13,14,16)/t5-,6+/m0/s1 | PDB
MMDB
UniProtKB/TrEMBL
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| Purchase
CHEMBL
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Similars
| Article
PubMed
| n/a | n/a | 855 | n/a | n/a | n/a | n/a | n/a | n/a |
Universidad de Alcal£
Curated by ChEMBL
| Assay Description
Inhibitory activity against wild-type HIV-1 reverse transcriptase |
J Med Chem 44: 1853-65 (2001)
Article DOI: 10.1021/jm001095i
BindingDB Entry DOI: 10.7270/Q20V8GJ7 |
More data for this
Ligand-Target Pair | |
Reverse transcriptase/RNaseH
(Human immunodeficiency virus 1) | BDBM50369390

(CHEMBL54224)Show SMILES Nc1nc2n(cnc2c(=O)[nH]1)[C@H]1CC[C@@H](CO[P@](O)(=O)O[P@@](O)(=O)OP(O)(O)=O)O1 |r| Show InChI InChI=1S/C10H16N5O12P3/c11-10-13-8-7(9(16)14-10)12-4-15(8)6-2-1-5(25-6)3-24-29(20,21)27-30(22,23)26-28(17,18)19/h4-6H,1-3H2,(H,20,21)(H,22,23)(H2,17,18,19)(H3,11,13,14,16)/t5-,6+/m0/s1 | PDB
MMDB
UniProtKB/TrEMBL
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| Purchase
CHEMBL
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Similars
| Article
PubMed
| n/a | n/a | 875 | n/a | n/a | n/a | n/a | n/a | n/a |
Universidad de Alcal£
Curated by ChEMBL
| Assay Description
Inhibitory activity against E138K mutant reverse transcriptase |
J Med Chem 44: 1853-65 (2001)
Article DOI: 10.1021/jm001095i
BindingDB Entry DOI: 10.7270/Q20V8GJ7 |
More data for this
Ligand-Target Pair | |
Reverse transcriptase/RNaseH
(Human immunodeficiency virus 1) | BDBM50369390

(CHEMBL54224)Show SMILES Nc1nc2n(cnc2c(=O)[nH]1)[C@H]1CC[C@@H](CO[P@](O)(=O)O[P@@](O)(=O)OP(O)(O)=O)O1 |r| Show InChI InChI=1S/C10H16N5O12P3/c11-10-13-8-7(9(16)14-10)12-4-15(8)6-2-1-5(25-6)3-24-29(20,21)27-30(22,23)26-28(17,18)19/h4-6H,1-3H2,(H,20,21)(H,22,23)(H2,17,18,19)(H3,11,13,14,16)/t5-,6+/m0/s1 | PDB
MMDB
UniProtKB/TrEMBL
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| Purchase
CHEMBL
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.09E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Universidad de Alcal£
Curated by ChEMBL
| Assay Description
Inhibitory activity against R172A mutant reverse transcriptase |
J Med Chem 44: 1853-65 (2001)
Article DOI: 10.1021/jm001095i
BindingDB Entry DOI: 10.7270/Q20V8GJ7 |
More data for this
Ligand-Target Pair | |
Reverse transcriptase/RNaseH
(Human immunodeficiency virus 1) | BDBM1944

(BHAP deriv. | CHEMBL593 | DELAVIRDINE MESYLATE | D...)Show SMILES CC(C)Nc1cccnc1N1CCN(CC1)C(=O)c1cc2cc(NS(C)(=O)=O)ccc2[nH]1 Show InChI InChI=1S/C22H28N6O3S/c1-15(2)24-19-5-4-8-23-21(19)27-9-11-28(12-10-27)22(29)20-14-16-13-17(26-32(3,30)31)6-7-18(16)25-20/h4-8,13-15,24-26H,9-12H2,1-3H3 | PDB
MMDB
UniProtKB/TrEMBL
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| DrugBank
MMDB
PDB
Article
PubMed
| n/a | n/a | 4.38E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Universidad de Alcal£
Curated by ChEMBL
| Assay Description
Inhibitory activity against E138K mutant reverse transcriptase |
J Med Chem 44: 1853-65 (2001)
Article DOI: 10.1021/jm001095i
BindingDB Entry DOI: 10.7270/Q20V8GJ7 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Reverse transcriptase/RNaseH
(Human immunodeficiency virus 1) | BDBM50192289

(1-[(5R,6R,8R,9R)-4-amino-9-(tert-butyl-dimethyl-si...)Show SMILES Cc1cn([C@@H]2O[C@H](CO[Si](C)(C)C(C)(C)C)[C@@]3(OS(=O)(=O)C=C3N)[C@H]2O[Si](C)(C)C(C)(C)C)c(=O)n(C)c1=O |c:21| Show InChI InChI=1S/C25H45N3O8SSi2/c1-16-13-28(22(30)27(8)20(16)29)21-19(35-39(11,12)24(5,6)7)25(17(26)15-37(31,32)36-25)18(34-21)14-33-38(9,10)23(2,3)4/h13,15,18-19,21H,14,26H2,1-12H3/t18-,19+,21-,25-/m1/s1 | PDB
MMDB
UniProtKB/TrEMBL
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 4.63E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Universidad de Alcal£
Curated by ChEMBL
| Assay Description
Inhibitory activity against wild-type HIV-1 reverse transcriptase |
J Med Chem 44: 1853-65 (2001)
Article DOI: 10.1021/jm001095i
BindingDB Entry DOI: 10.7270/Q20V8GJ7 |
More data for this
Ligand-Target Pair | |
Reverse transcriptase/RNaseH
(Human immunodeficiency virus 1) | BDBM50473243

(CHEMBL2115411)Show SMILES Cc1cn([C@@H]2O[C@H](CO[Si](C)(C)C(C)(C)C)C3(OS(=O)(=O)C=C3N)[C@H]2O[Si](C)(C)C(C)(C)C)c(=O)[nH]c1=O |r,c:21| Show InChI InChI=1S/C24H43N3O8SSi2/c1-15-12-27(21(29)26-19(15)28)20-18(34-38(10,11)23(5,6)7)24(16(25)14-36(30,31)35-24)17(33-20)13-32-37(8,9)22(2,3)4/h12,14,17-18,20H,13,25H2,1-11H3,(H,26,28,29)/t17-,18+,20-,24?/m1/s1 | PDB
MMDB
UniProtKB/TrEMBL
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 6.10E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Universidad de Alcal£
Curated by ChEMBL
| Assay Description
Inhibitory activity against wild-type HIV-1 reverse transcriptase |
J Med Chem 44: 1853-65 (2001)
Article DOI: 10.1021/jm001095i
BindingDB Entry DOI: 10.7270/Q20V8GJ7 |
More data for this
Ligand-Target Pair | |
Reverse transcriptase/RNaseH
(Human immunodeficiency virus 1) | BDBM50192289

(1-[(5R,6R,8R,9R)-4-amino-9-(tert-butyl-dimethyl-si...)Show SMILES Cc1cn([C@@H]2O[C@H](CO[Si](C)(C)C(C)(C)C)[C@@]3(OS(=O)(=O)C=C3N)[C@H]2O[Si](C)(C)C(C)(C)C)c(=O)n(C)c1=O |c:21| Show InChI InChI=1S/C25H45N3O8SSi2/c1-16-13-28(22(30)27(8)20(16)29)21-19(35-39(11,12)24(5,6)7)25(17(26)15-37(31,32)36-25)18(34-21)14-33-38(9,10)23(2,3)4/h13,15,18-19,21H,14,26H2,1-12H3/t18-,19+,21-,25-/m1/s1 | PDB
MMDB
UniProtKB/TrEMBL
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | >8.27E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Universidad de Alcal£
Curated by ChEMBL
| Assay Description
Inhibitory activity against E138K mutant reverse transcriptase |
J Med Chem 44: 1853-65 (2001)
Article DOI: 10.1021/jm001095i
BindingDB Entry DOI: 10.7270/Q20V8GJ7 |
More data for this
Ligand-Target Pair | |
Reverse transcriptase/RNaseH
(Human immunodeficiency virus 1) | BDBM50473243

(CHEMBL2115411)Show SMILES Cc1cn([C@@H]2O[C@H](CO[Si](C)(C)C(C)(C)C)C3(OS(=O)(=O)C=C3N)[C@H]2O[Si](C)(C)C(C)(C)C)c(=O)[nH]c1=O |r,c:21| Show InChI InChI=1S/C24H43N3O8SSi2/c1-15-12-27(21(29)26-19(15)28)20-18(34-38(10,11)23(5,6)7)24(16(25)14-36(30,31)35-24)17(33-20)13-32-37(8,9)22(2,3)4/h12,14,17-18,20H,13,25H2,1-11H3,(H,26,28,29)/t17-,18+,20-,24?/m1/s1 | PDB
MMDB
UniProtKB/TrEMBL
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | >8.47E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Universidad de Alcal£
Curated by ChEMBL
| Assay Description
Inhibitory activity against E138K mutant reverse transcriptase |
J Med Chem 44: 1853-65 (2001)
Article DOI: 10.1021/jm001095i
BindingDB Entry DOI: 10.7270/Q20V8GJ7 |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data