

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| UDP-3-O-acyl-N-acetylglucosamine deacetylase (Escherichia coli) | BDBM50200120 (CHEMBL260091 | CHIR-090 | US10875832, Compound ChI...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid PDB UniChem | Article PubMed | 8 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Institut f£r Pharmazeutische und Medizinische Chemie der Westf£lischen Wilhelms-Universit£t M£nster Curated by ChEMBL | Assay Description Inhibition of Escherichia coli LpxC using UDP-3-O-[(R)-3-hydroxymyristoyl]-N-acetylglucosamine as substrate incubated for 30 mins prior to enzyme add... | Bioorg Med Chem 22: 1016-28 (2014) Article DOI: 10.1016/j.bmc.2013.12.057 BindingDB Entry DOI: 10.7270/Q2X92F7C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| UDP-3-O-acyl-N-acetylglucosamine deacetylase (Escherichia coli) | BDBM50495314 (CHEMBL3103559) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 66 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Institut f£r Pharmazeutische und Medizinische Chemie der Westf£lischen Wilhelms-Universit£t M£nster Curated by ChEMBL | Assay Description Inhibition of Escherichia coli LpxC using UDP-3-O-[(R)-3-hydroxymyristoyl]-N-acetylglucosamine as substrate incubated for 30 mins prior to enzyme add... | Bioorg Med Chem 22: 1016-28 (2014) Article DOI: 10.1016/j.bmc.2013.12.057 BindingDB Entry DOI: 10.7270/Q2X92F7C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| UDP-3-O-acyl-N-acetylglucosamine deacetylase (Escherichia coli) | BDBM50495317 (CHEMBL3103561) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 95 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Institut f£r Pharmazeutische und Medizinische Chemie der Westf£lischen Wilhelms-Universit£t M£nster Curated by ChEMBL | Assay Description Inhibition of Escherichia coli LpxC using UDP-3-O-[(R)-3-hydroxymyristoyl]-N-acetylglucosamine as substrate incubated for 30 mins prior to enzyme add... | Bioorg Med Chem 22: 1016-28 (2014) Article DOI: 10.1016/j.bmc.2013.12.057 BindingDB Entry DOI: 10.7270/Q2X92F7C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| UDP-3-O-acyl-N-acetylglucosamine deacetylase (Escherichia coli) | BDBM50495323 (CHEMBL3103548) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 358 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Institut f£r Pharmazeutische und Medizinische Chemie der Westf£lischen Wilhelms-Universit£t M£nster Curated by ChEMBL | Assay Description Inhibition of Escherichia coli LpxC using UDP-3-O-[(R)-3-hydroxymyristoyl]-N-acetylglucosamine as substrate incubated for 30 mins prior to enzyme add... | Bioorg Med Chem 22: 1016-28 (2014) Article DOI: 10.1016/j.bmc.2013.12.057 BindingDB Entry DOI: 10.7270/Q2X92F7C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| UDP-3-O-acyl-N-acetylglucosamine deacetylase (Escherichia coli) | BDBM50495312 (CHEMBL3103550) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 1.45E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Institut f£r Pharmazeutische und Medizinische Chemie der Westf£lischen Wilhelms-Universit£t M£nster Curated by ChEMBL | Assay Description Inhibition of Escherichia coli LpxC using UDP-3-O-[(R)-3-hydroxymyristoyl]-N-acetylglucosamine as substrate incubated for 30 mins prior to enzyme add... | Bioorg Med Chem 22: 1016-28 (2014) Article DOI: 10.1016/j.bmc.2013.12.057 BindingDB Entry DOI: 10.7270/Q2X92F7C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| UDP-3-O-acyl-N-acetylglucosamine deacetylase (Escherichia coli) | BDBM50495316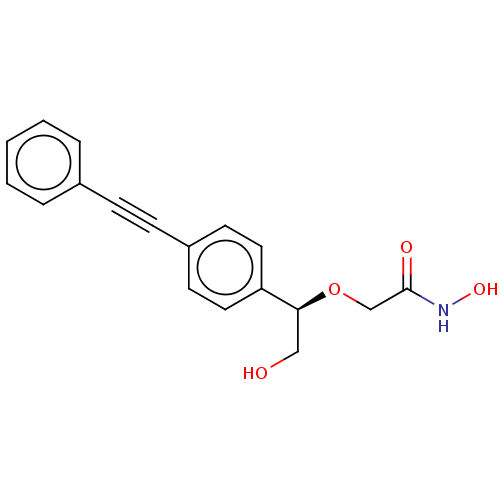 (CHEMBL3103560) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 4.40E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Institut f£r Pharmazeutische und Medizinische Chemie der Westf£lischen Wilhelms-Universit£t M£nster Curated by ChEMBL | Assay Description Inhibition of Escherichia coli LpxC using UDP-3-O-[(R)-3-hydroxymyristoyl]-N-acetylglucosamine as substrate incubated for 30 mins prior to enzyme add... | Bioorg Med Chem 22: 1016-28 (2014) Article DOI: 10.1016/j.bmc.2013.12.057 BindingDB Entry DOI: 10.7270/Q2X92F7C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| UDP-3-O-acyl-N-acetylglucosamine deacetylase (Escherichia coli) | BDBM50495311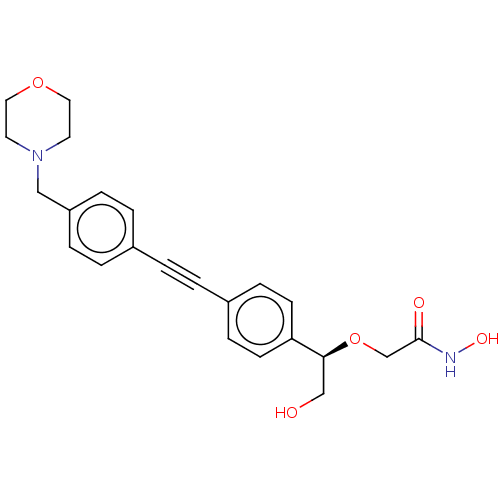 (CHEMBL3103562) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 2.73E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Institut f£r Pharmazeutische und Medizinische Chemie der Westf£lischen Wilhelms-Universit£t M£nster Curated by ChEMBL | Assay Description Inhibition of Escherichia coli LpxC using UDP-3-O-[(R)-3-hydroxymyristoyl]-N-acetylglucosamine as substrate incubated for 30 mins prior to enzyme add... | Bioorg Med Chem 22: 1016-28 (2014) Article DOI: 10.1016/j.bmc.2013.12.057 BindingDB Entry DOI: 10.7270/Q2X92F7C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| UDP-3-O-acyl-N-acetylglucosamine deacetylase (Escherichia coli) | BDBM50200120 (CHEMBL260091 | CHIR-090 | US10875832, Compound ChI...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid PDB UniChem | Article PubMed | n/a | n/a | 58 | n/a | n/a | n/a | n/a | n/a | n/a |
Institut f£r Pharmazeutische und Medizinische Chemie der Westf£lischen Wilhelms-Universit£t M£nster Curated by ChEMBL | Assay Description Inhibition of Escherichia coli LpxC using UDP-3-O-[(R)-3-hydroxymyristoyl]-N-acetylglucosamine as substrate incubated for 30 mins prior to enzyme add... | Bioorg Med Chem 22: 1016-28 (2014) Article DOI: 10.1016/j.bmc.2013.12.057 BindingDB Entry DOI: 10.7270/Q2X92F7C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| UDP-3-O-acyl-N-acetylglucosamine deacetylase (Escherichia coli) | BDBM50495314 (CHEMBL3103559) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 480 | n/a | n/a | n/a | n/a | n/a | n/a |
Institut f£r Pharmazeutische und Medizinische Chemie der Westf£lischen Wilhelms-Universit£t M£nster Curated by ChEMBL | Assay Description Inhibition of Escherichia coli LpxC using UDP-3-O-[(R)-3-hydroxymyristoyl]-N-acetylglucosamine as substrate incubated for 30 mins prior to enzyme add... | Bioorg Med Chem 22: 1016-28 (2014) Article DOI: 10.1016/j.bmc.2013.12.057 BindingDB Entry DOI: 10.7270/Q2X92F7C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| UDP-3-O-acyl-N-acetylglucosamine deacetylase (Escherichia coli) | BDBM50495317 (CHEMBL3103561) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 690 | n/a | n/a | n/a | n/a | n/a | n/a |
Institut f£r Pharmazeutische und Medizinische Chemie der Westf£lischen Wilhelms-Universit£t M£nster Curated by ChEMBL | Assay Description Inhibition of Escherichia coli LpxC using UDP-3-O-[(R)-3-hydroxymyristoyl]-N-acetylglucosamine as substrate incubated for 30 mins prior to enzyme add... | Bioorg Med Chem 22: 1016-28 (2014) Article DOI: 10.1016/j.bmc.2013.12.057 BindingDB Entry DOI: 10.7270/Q2X92F7C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| UDP-3-O-acyl-N-acetylglucosamine deacetylase (Escherichia coli) | BDBM50495323 (CHEMBL3103548) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 2.60E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Institut f£r Pharmazeutische und Medizinische Chemie der Westf£lischen Wilhelms-Universit£t M£nster Curated by ChEMBL | Assay Description Inhibition of Escherichia coli LpxC using UDP-3-O-[(R)-3-hydroxymyristoyl]-N-acetylglucosamine as substrate incubated for 30 mins prior to enzyme add... | Bioorg Med Chem 22: 1016-28 (2014) Article DOI: 10.1016/j.bmc.2013.12.057 BindingDB Entry DOI: 10.7270/Q2X92F7C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| UDP-3-O-acyl-N-acetylglucosamine deacetylase (Escherichia coli) | BDBM50495312 (CHEMBL3103550) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 1.05E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Institut f£r Pharmazeutische und Medizinische Chemie der Westf£lischen Wilhelms-Universit£t M£nster Curated by ChEMBL | Assay Description Inhibition of Escherichia coli LpxC using UDP-3-O-[(R)-3-hydroxymyristoyl]-N-acetylglucosamine as substrate incubated for 30 mins prior to enzyme add... | Bioorg Med Chem 22: 1016-28 (2014) Article DOI: 10.1016/j.bmc.2013.12.057 BindingDB Entry DOI: 10.7270/Q2X92F7C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| UDP-3-O-acyl-N-acetylglucosamine deacetylase (Escherichia coli) | BDBM50495316 (CHEMBL3103560) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 3.16E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Institut f£r Pharmazeutische und Medizinische Chemie der Westf£lischen Wilhelms-Universit£t M£nster Curated by ChEMBL | Assay Description Inhibition of Escherichia coli LpxC using UDP-3-O-[(R)-3-hydroxymyristoyl]-N-acetylglucosamine as substrate incubated for 30 mins prior to enzyme add... | Bioorg Med Chem 22: 1016-28 (2014) Article DOI: 10.1016/j.bmc.2013.12.057 BindingDB Entry DOI: 10.7270/Q2X92F7C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| UDP-3-O-acyl-N-acetylglucosamine deacetylase (Escherichia coli) | BDBM50495311 (CHEMBL3103562) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 1.98E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Institut f£r Pharmazeutische und Medizinische Chemie der Westf£lischen Wilhelms-Universit£t M£nster Curated by ChEMBL | Assay Description Inhibition of Escherichia coli LpxC using UDP-3-O-[(R)-3-hydroxymyristoyl]-N-acetylglucosamine as substrate incubated for 30 mins prior to enzyme add... | Bioorg Med Chem 22: 1016-28 (2014) Article DOI: 10.1016/j.bmc.2013.12.057 BindingDB Entry DOI: 10.7270/Q2X92F7C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| UDP-3-O-acyl-N-acetylglucosamine deacetylase (Escherichia coli) | BDBM50495325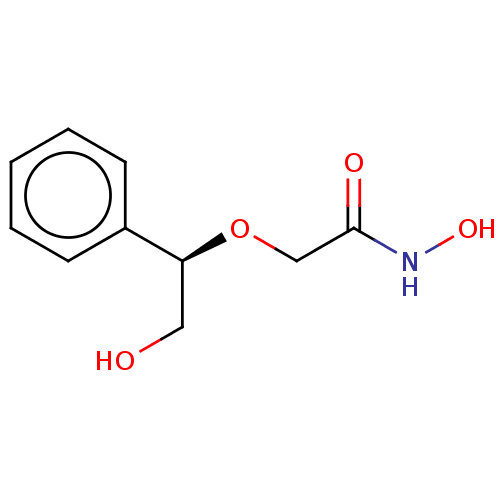 (CHEMBL3103552) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | >2.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Institut f£r Pharmazeutische und Medizinische Chemie der Westf£lischen Wilhelms-Universit£t M£nster Curated by ChEMBL | Assay Description Inhibition of Escherichia coli LpxC using UDP-3-O-[(R)-3-hydroxymyristoyl]-N-acetylglucosamine as substrate incubated for 30 mins prior to enzyme add... | Bioorg Med Chem 22: 1016-28 (2014) Article DOI: 10.1016/j.bmc.2013.12.057 BindingDB Entry DOI: 10.7270/Q2X92F7C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| UDP-3-O-acyl-N-acetylglucosamine deacetylase (Escherichia coli) | BDBM50495324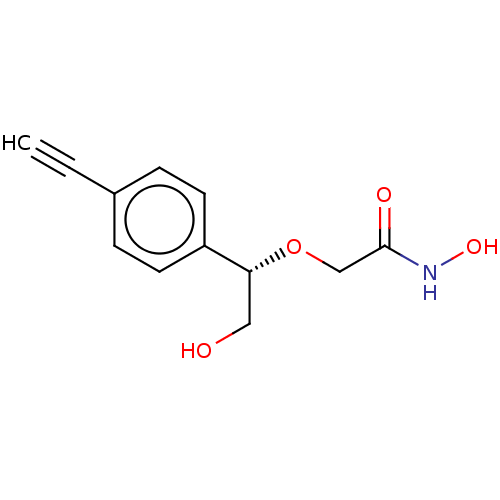 (CHEMBL3103555) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | >2.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Institut f£r Pharmazeutische und Medizinische Chemie der Westf£lischen Wilhelms-Universit£t M£nster Curated by ChEMBL | Assay Description Inhibition of Escherichia coli LpxC using UDP-3-O-[(R)-3-hydroxymyristoyl]-N-acetylglucosamine as substrate incubated for 30 mins prior to enzyme add... | Bioorg Med Chem 22: 1016-28 (2014) Article DOI: 10.1016/j.bmc.2013.12.057 BindingDB Entry DOI: 10.7270/Q2X92F7C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| UDP-3-O-acyl-N-acetylglucosamine deacetylase (Escherichia coli) | BDBM50495322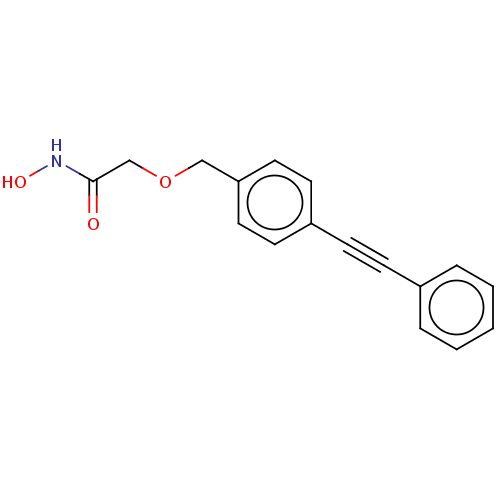 (CHEMBL3103549) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | >2.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Institut f£r Pharmazeutische und Medizinische Chemie der Westf£lischen Wilhelms-Universit£t M£nster Curated by ChEMBL | Assay Description Inhibition of Escherichia coli LpxC using UDP-3-O-[(R)-3-hydroxymyristoyl]-N-acetylglucosamine as substrate incubated for 30 mins prior to enzyme add... | Bioorg Med Chem 22: 1016-28 (2014) Article DOI: 10.1016/j.bmc.2013.12.057 BindingDB Entry DOI: 10.7270/Q2X92F7C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| UDP-3-O-acyl-N-acetylglucosamine deacetylase (Escherichia coli) | BDBM50495321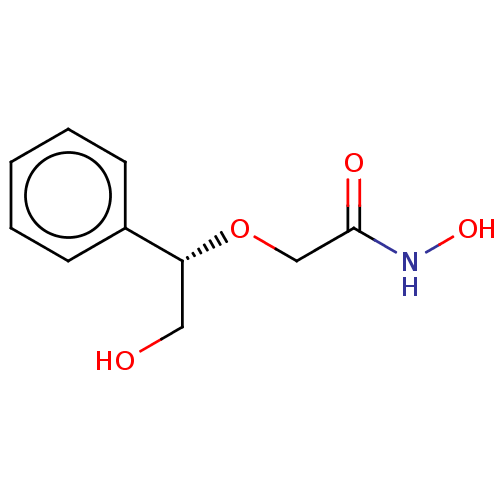 (CHEMBL3103551) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | >2.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Institut f£r Pharmazeutische und Medizinische Chemie der Westf£lischen Wilhelms-Universit£t M£nster Curated by ChEMBL | Assay Description Inhibition of Escherichia coli LpxC using UDP-3-O-[(R)-3-hydroxymyristoyl]-N-acetylglucosamine as substrate incubated for 30 mins prior to enzyme add... | Bioorg Med Chem 22: 1016-28 (2014) Article DOI: 10.1016/j.bmc.2013.12.057 BindingDB Entry DOI: 10.7270/Q2X92F7C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| UDP-3-O-acyl-N-acetylglucosamine deacetylase (Escherichia coli) | BDBM50495320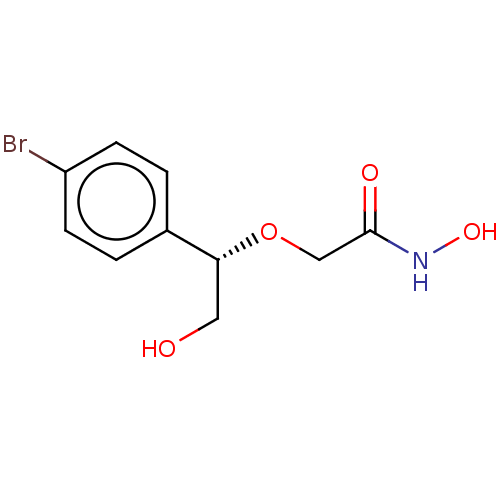 (CHEMBL3103553) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | >2.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Institut f£r Pharmazeutische und Medizinische Chemie der Westf£lischen Wilhelms-Universit£t M£nster Curated by ChEMBL | Assay Description Inhibition of Escherichia coli LpxC using UDP-3-O-[(R)-3-hydroxymyristoyl]-N-acetylglucosamine as substrate incubated for 30 mins prior to enzyme add... | Bioorg Med Chem 22: 1016-28 (2014) Article DOI: 10.1016/j.bmc.2013.12.057 BindingDB Entry DOI: 10.7270/Q2X92F7C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| UDP-3-O-acyl-N-acetylglucosamine deacetylase (Escherichia coli) | BDBM50495319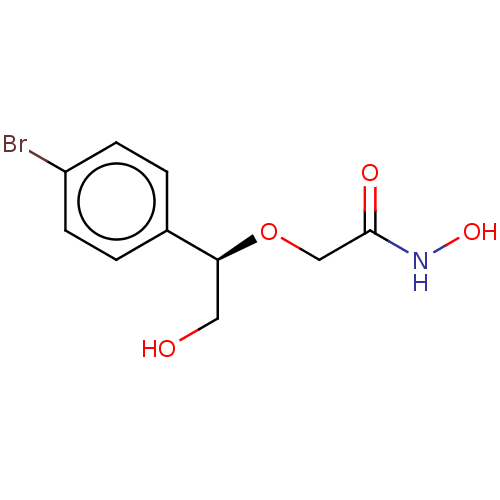 (CHEMBL3103554) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | >2.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Institut f£r Pharmazeutische und Medizinische Chemie der Westf£lischen Wilhelms-Universit£t M£nster Curated by ChEMBL | Assay Description Inhibition of Escherichia coli LpxC using UDP-3-O-[(R)-3-hydroxymyristoyl]-N-acetylglucosamine as substrate incubated for 30 mins prior to enzyme add... | Bioorg Med Chem 22: 1016-28 (2014) Article DOI: 10.1016/j.bmc.2013.12.057 BindingDB Entry DOI: 10.7270/Q2X92F7C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| UDP-3-O-acyl-N-acetylglucosamine deacetylase (Escherichia coli) | BDBM50495326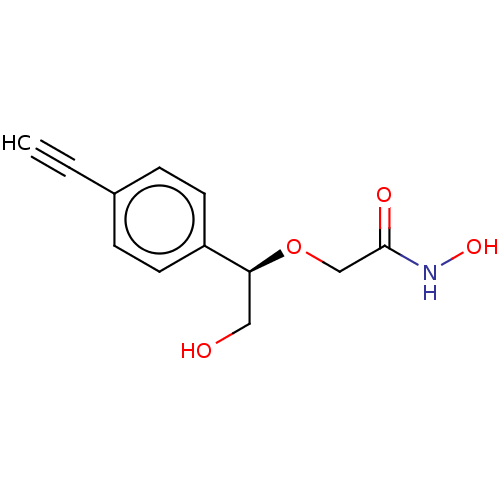 (CHEMBL3103556) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | >2.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Institut f£r Pharmazeutische und Medizinische Chemie der Westf£lischen Wilhelms-Universit£t M£nster Curated by ChEMBL | Assay Description Inhibition of Escherichia coli LpxC using UDP-3-O-[(R)-3-hydroxymyristoyl]-N-acetylglucosamine as substrate incubated for 30 mins prior to enzyme add... | Bioorg Med Chem 22: 1016-28 (2014) Article DOI: 10.1016/j.bmc.2013.12.057 BindingDB Entry DOI: 10.7270/Q2X92F7C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| UDP-3-O-acyl-N-acetylglucosamine deacetylase (Escherichia coli) | BDBM50495315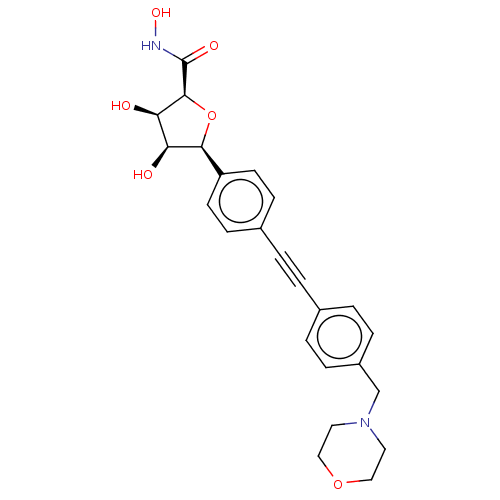 (CHEMBL3103547) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | >2.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Institut f£r Pharmazeutische und Medizinische Chemie der Westf£lischen Wilhelms-Universit£t M£nster Curated by ChEMBL | Assay Description Inhibition of Escherichia coli LpxC using UDP-3-O-[(R)-3-hydroxymyristoyl]-N-acetylglucosamine as substrate incubated for 30 mins prior to enzyme add... | Bioorg Med Chem 22: 1016-28 (2014) Article DOI: 10.1016/j.bmc.2013.12.057 BindingDB Entry DOI: 10.7270/Q2X92F7C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| UDP-3-O-acyl-N-acetylglucosamine deacetylase (Escherichia coli) | BDBM50495313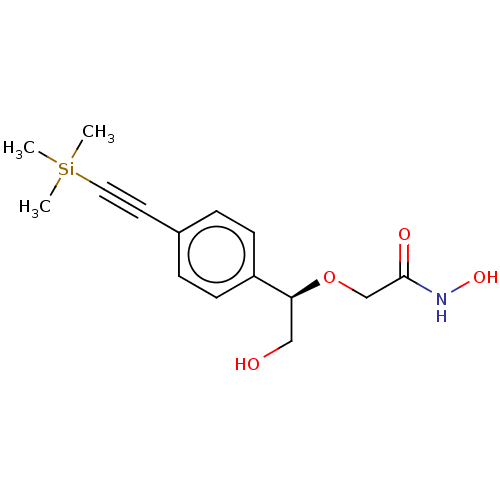 (CHEMBL3103558) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | >2.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Institut f£r Pharmazeutische und Medizinische Chemie der Westf£lischen Wilhelms-Universit£t M£nster Curated by ChEMBL | Assay Description Inhibition of Escherichia coli LpxC using UDP-3-O-[(R)-3-hydroxymyristoyl]-N-acetylglucosamine as substrate incubated for 30 mins prior to enzyme add... | Bioorg Med Chem 22: 1016-28 (2014) Article DOI: 10.1016/j.bmc.2013.12.057 BindingDB Entry DOI: 10.7270/Q2X92F7C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| UDP-3-O-acyl-N-acetylglucosamine deacetylase (Escherichia coli) | BDBM50495318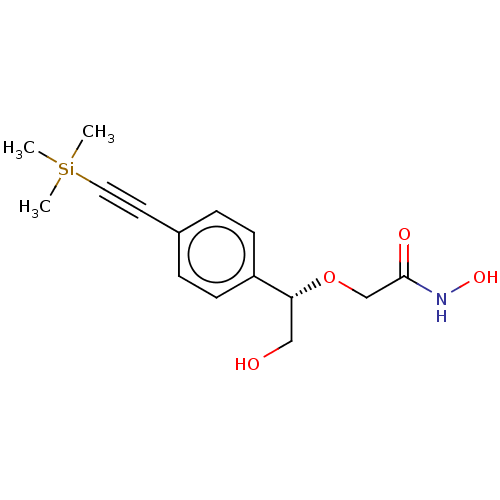 (CHEMBL3103557) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | >2.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Institut f£r Pharmazeutische und Medizinische Chemie der Westf£lischen Wilhelms-Universit£t M£nster Curated by ChEMBL | Assay Description Inhibition of Escherichia coli LpxC using UDP-3-O-[(R)-3-hydroxymyristoyl]-N-acetylglucosamine as substrate incubated for 30 mins prior to enzyme add... | Bioorg Med Chem 22: 1016-28 (2014) Article DOI: 10.1016/j.bmc.2013.12.057 BindingDB Entry DOI: 10.7270/Q2X92F7C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||