 Found 21 hits Enz. Inhib. hit(s) with all data for entry = 50007239
Found 21 hits Enz. Inhib. hit(s) with all data for entry = 50007239 Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Cytochrome P450 1B1
(Homo sapiens (Human)) | BDBM50108052
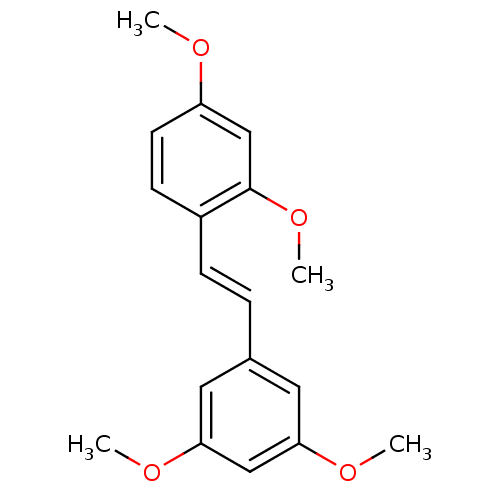
(1-[(E)-2-(3,5-dimethoxyphenyl)vinyl]-2,4-dimethoxy...)Show InChI InChI=1S/C18H20O4/c1-19-15-8-7-14(18(12-15)22-4)6-5-13-9-16(20-2)11-17(10-13)21-3/h5-12H,1-4H3/b6-5+ | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Birla Institute of Technology
Curated by ChEMBL
| Assay Description
Inhibition of human CYP1B1 using ethoxyresorufin as substrate preincubated for 3 mins followed by NADPH addition measured after 10 mins by EROD assay |
Eur J Med Chem 163: 28-36 (2019)
Article DOI: 10.1016/j.ejmech.2018.11.039
BindingDB Entry DOI: 10.7270/Q2930XFF |
More data for this
Ligand-Target Pair | |
Cytochrome P450 1B1
(Homo sapiens (Human)) | BDBM23926

((E)-resveratrol | 5-[(E)-2-(4-hydroxyphenyl)etheny...)Show InChI InChI=1S/C14H12O3/c15-12-5-3-10(4-6-12)1-2-11-7-13(16)9-14(17)8-11/h1-9,15-17H/b2-1+ | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| 750 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Birla Institute of Technology
Curated by ChEMBL
| Assay Description
Mixed type inhibition of human CYP1B1 by EROD assay |
Eur J Med Chem 163: 28-36 (2019)
Article DOI: 10.1016/j.ejmech.2018.11.039
BindingDB Entry DOI: 10.7270/Q2930XFF |
More data for this
Ligand-Target Pair | |
Cytochrome P450 1B1
(Homo sapiens (Human)) | BDBM23409

(3,5,7-trihydroxy-2-(4-hydroxy-3-methoxyphenyl)-4H-...)Show InChI InChI=1S/C16H12O7/c1-22-11-4-7(2-3-9(11)18)16-15(21)14(20)13-10(19)5-8(17)6-12(13)23-16/h2-6,17-19,21H,1H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
KEGG
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| 3.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Birla Institute of Technology
Curated by ChEMBL
| Assay Description
Inhibition of human liver microsomes CYP1B1 expressed in Escherichia coli DH5alpha cell membranes coexpressing human NADPH-cytochrome P450 reductase ... |
Eur J Med Chem 163: 28-36 (2019)
Article DOI: 10.1016/j.ejmech.2018.11.039
BindingDB Entry DOI: 10.7270/Q2930XFF |
More data for this
Ligand-Target Pair | |
Cytochrome P450 1B1
(Homo sapiens (Human)) | BDBM50081711
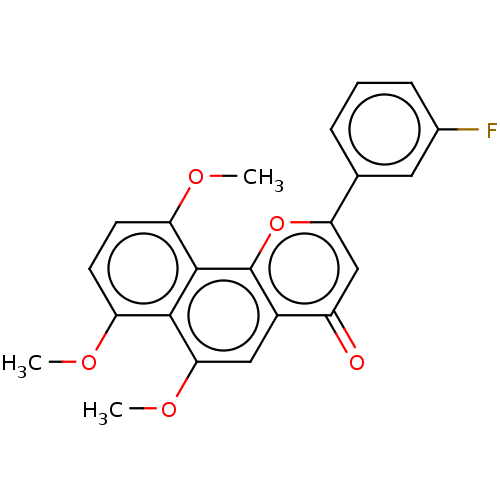
(CHEMBL3421639)Show SMILES COc1ccc(OC)c2c3oc(cc(=O)c3cc(OC)c12)-c1cccc(F)c1 Show InChI InChI=1S/C22H17FO5/c1-25-16-7-8-17(26-2)21-20(16)19(27-3)10-14-15(24)11-18(28-22(14)21)12-5-4-6-13(23)9-12/h4-11H,1-3H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.0430 | n/a | n/a | n/a | n/a | n/a | n/a |
Birla Institute of Technology
Curated by ChEMBL
| Assay Description
Inhibition of human CYP1B1 by EROD assay |
Eur J Med Chem 163: 28-36 (2019)
Article DOI: 10.1016/j.ejmech.2018.11.039
BindingDB Entry DOI: 10.7270/Q2930XFF |
More data for this
Ligand-Target Pair | |
Cytochrome P450 1A1
(Homo sapiens (Human)) | BDBM50507600
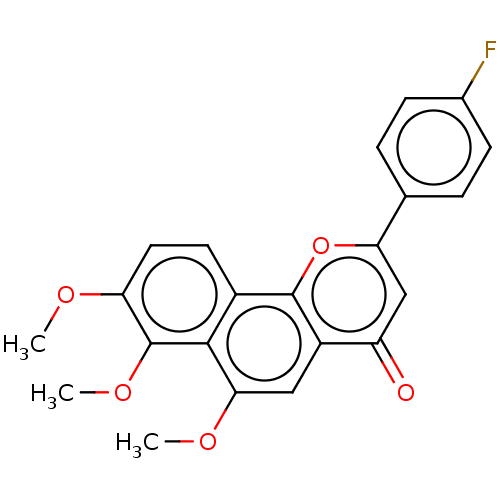
(CHEMBL4467401)Show SMILES COc1ccc2c3oc(cc(=O)c3cc(OC)c2c1OC)-c1ccc(F)cc1 Show InChI InChI=1S/C22H17FO5/c1-25-17-9-8-14-20(22(17)27-3)19(26-2)10-15-16(24)11-18(28-21(14)15)12-4-6-13(23)7-5-12/h4-11H,1-3H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Birla Institute of Technology
Curated by ChEMBL
| Assay Description
Inhibition of human CYP1A1 by EROD assay |
Eur J Med Chem 163: 28-36 (2019)
Article DOI: 10.1016/j.ejmech.2018.11.039
BindingDB Entry DOI: 10.7270/Q2930XFF |
More data for this
Ligand-Target Pair | |
Cytochrome P450 1B1
(Homo sapiens (Human)) | BDBM50507596
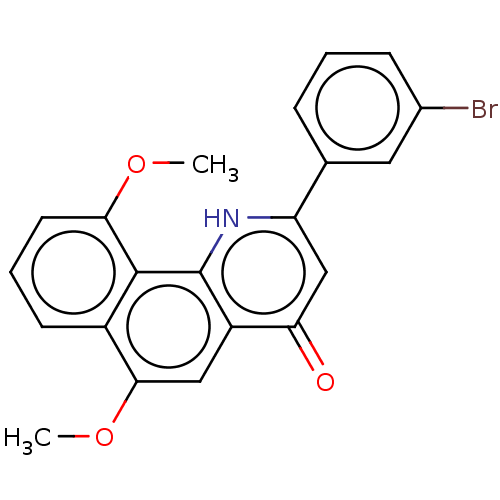
(CHEMBL4532099)Show SMILES COc1cccc2c(OC)cc3c([nH]c(cc3=O)-c3cccc(Br)c3)c12 Show InChI InChI=1S/C21H16BrNO3/c1-25-18-8-4-7-14-19(26-2)10-15-17(24)11-16(23-21(15)20(14)18)12-5-3-6-13(22)9-12/h3-11H,1-2H3,(H,23,24) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 3.60 | n/a | n/a | n/a | n/a | n/a | n/a |
Birla Institute of Technology
Curated by ChEMBL
| Assay Description
Inhibition of human CYP1B1 using 7-Ethoxyresorufin as substrate after 30 mins in presence of NADP+ by EROD assay |
Eur J Med Chem 163: 28-36 (2019)
Article DOI: 10.1016/j.ejmech.2018.11.039
BindingDB Entry DOI: 10.7270/Q2930XFF |
More data for this
Ligand-Target Pair | |
Cytochrome P450 1B1
(Homo sapiens (Human)) | BDBM50507598
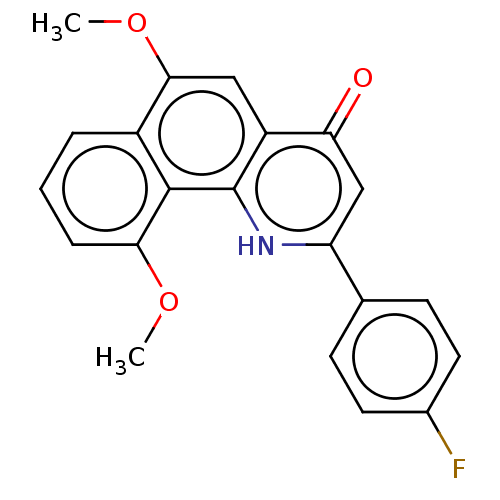
(CHEMBL4539787)Show SMILES COc1cc2c([nH]c(cc2=O)-c2ccc(F)cc2)c2c(OC)cccc12 Show InChI InChI=1S/C21H16FNO3/c1-25-18-5-3-4-14-19(26-2)10-15-17(24)11-16(23-21(15)20(14)18)12-6-8-13(22)9-7-12/h3-11H,1-2H3,(H,23,24) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 3.90 | n/a | n/a | n/a | n/a | n/a | n/a |
Birla Institute of Technology
Curated by ChEMBL
| Assay Description
Inhibition of human CYP1B1 using 7-Ethoxyresorufin as substrate after 30 mins in presence of NADP+ by EROD assay |
Eur J Med Chem 163: 28-36 (2019)
Article DOI: 10.1016/j.ejmech.2018.11.039
BindingDB Entry DOI: 10.7270/Q2930XFF |
More data for this
Ligand-Target Pair | |
Cytochrome P450 1B1
(Homo sapiens (Human)) | BDBM50507599
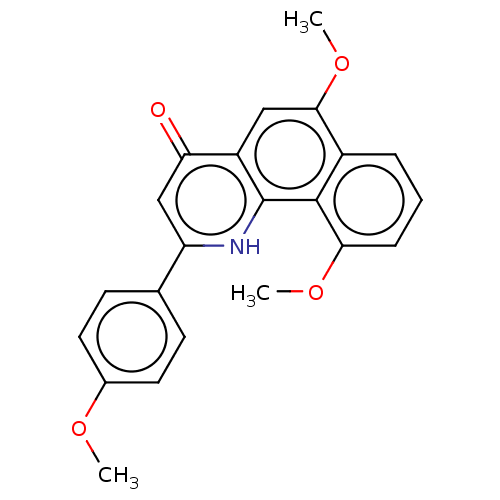
(CHEMBL4456952)Show SMILES COc1ccc(cc1)-c1cc(=O)c2cc(OC)c3cccc(OC)c3c2[nH]1 Show InChI InChI=1S/C22H19NO4/c1-25-14-9-7-13(8-10-14)17-12-18(24)16-11-20(27-3)15-5-4-6-19(26-2)21(15)22(16)23-17/h4-12H,1-3H3,(H,23,24) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 4.10 | n/a | n/a | n/a | n/a | n/a | n/a |
Birla Institute of Technology
Curated by ChEMBL
| Assay Description
Inhibition of human CYP1B1 using 7-Ethoxyresorufin as substrate after 30 mins in presence of NADP+ by EROD assay |
Eur J Med Chem 163: 28-36 (2019)
Article DOI: 10.1016/j.ejmech.2018.11.039
BindingDB Entry DOI: 10.7270/Q2930XFF |
More data for this
Ligand-Target Pair | |
Cytochrome P450 1A1
(Homo sapiens (Human)) | BDBM50507598

(CHEMBL4539787)Show SMILES COc1cc2c([nH]c(cc2=O)-c2ccc(F)cc2)c2c(OC)cccc12 Show InChI InChI=1S/C21H16FNO3/c1-25-18-5-3-4-14-19(26-2)10-15-17(24)11-16(23-21(15)20(14)18)12-6-8-13(22)9-7-12/h3-11H,1-2H3,(H,23,24) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 11 | n/a | n/a | n/a | n/a | n/a | n/a |
Birla Institute of Technology
Curated by ChEMBL
| Assay Description
Inhibition of human CYP1A1 using 7-Ethoxyresorufin as substrate after 15 mins in presence of NADP+ by EROD assay |
Eur J Med Chem 163: 28-36 (2019)
Article DOI: 10.1016/j.ejmech.2018.11.039
BindingDB Entry DOI: 10.7270/Q2930XFF |
More data for this
Ligand-Target Pair | |
Cytochrome P450 1A1
(Homo sapiens (Human)) | BDBM50507596

(CHEMBL4532099)Show SMILES COc1cccc2c(OC)cc3c([nH]c(cc3=O)-c3cccc(Br)c3)c12 Show InChI InChI=1S/C21H16BrNO3/c1-25-18-8-4-7-14-19(26-2)10-15-17(24)11-16(23-21(15)20(14)18)12-5-3-6-13(22)9-12/h3-11H,1-2H3,(H,23,24) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 20 | n/a | n/a | n/a | n/a | n/a | n/a |
Birla Institute of Technology
Curated by ChEMBL
| Assay Description
Inhibition of human CYP1A1 using 7-Ethoxyresorufin as substrate after 15 mins in presence of NADP+ by EROD assay |
Eur J Med Chem 163: 28-36 (2019)
Article DOI: 10.1016/j.ejmech.2018.11.039
BindingDB Entry DOI: 10.7270/Q2930XFF |
More data for this
Ligand-Target Pair | |
Cytochrome P450 1A1
(Homo sapiens (Human)) | BDBM50507599

(CHEMBL4456952)Show SMILES COc1ccc(cc1)-c1cc(=O)c2cc(OC)c3cccc(OC)c3c2[nH]1 Show InChI InChI=1S/C22H19NO4/c1-25-14-9-7-13(8-10-14)17-12-18(24)16-11-20(27-3)15-5-4-6-19(26-2)21(15)22(16)23-17/h4-12H,1-3H3,(H,23,24) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 65 | n/a | n/a | n/a | n/a | n/a | n/a |
Birla Institute of Technology
Curated by ChEMBL
| Assay Description
Inhibition of human CYP1A1 using 7-Ethoxyresorufin as substrate after 15 mins in presence of NADP+ by EROD assay |
Eur J Med Chem 163: 28-36 (2019)
Article DOI: 10.1016/j.ejmech.2018.11.039
BindingDB Entry DOI: 10.7270/Q2930XFF |
More data for this
Ligand-Target Pair | |
Cytochrome P450 1B1
(Homo sapiens (Human)) | BDBM50203126
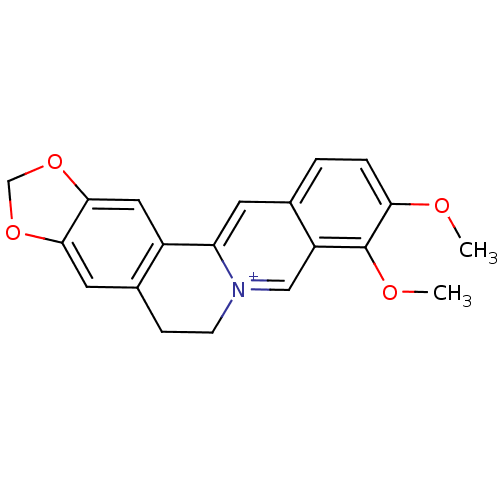
(3,4-dimethoxy-6,7-dihydro-[1,3]dioxolo[4,5-g]pyrid...)Show InChI InChI=1S/C20H18NO4/c1-22-17-4-3-12-7-16-14-9-19-18(24-11-25-19)8-13(14)5-6-21(16)10-15(12)20(17)23-2/h3-4,7-10H,5-6,11H2,1-2H3/q+1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
PC cid
PC sid
PDB
UniChem
Similars
| Article
PubMed
| n/a | n/a | 94 | n/a | n/a | n/a | n/a | n/a | n/a |
Birla Institute of Technology
Curated by ChEMBL
| Assay Description
Inhibition of human CYP1B1 expressed in Escherichia coli DH5alpha cell membranes coexpressing human NADPH-cytochrome P450 reductase using 7-Ethoxyres... |
Eur J Med Chem 163: 28-36 (2019)
Article DOI: 10.1016/j.ejmech.2018.11.039
BindingDB Entry DOI: 10.7270/Q2930XFF |
More data for this
Ligand-Target Pair | |
Cytochrome P450 1A2
(Homo sapiens (Human)) | BDBM50507598

(CHEMBL4539787)Show SMILES COc1cc2c([nH]c(cc2=O)-c2ccc(F)cc2)c2c(OC)cccc12 Show InChI InChI=1S/C21H16FNO3/c1-25-18-5-3-4-14-19(26-2)10-15-17(24)11-16(23-21(15)20(14)18)12-6-8-13(22)9-7-12/h3-11H,1-2H3,(H,23,24) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 190 | n/a | n/a | n/a | n/a | n/a | n/a |
Birla Institute of Technology
Curated by ChEMBL
| Assay Description
Inhibition of human CYP1A2 using 7-Ethoxyresorufin as substrate after 50 mins in presence of NADP+ by EROD assay |
Eur J Med Chem 163: 28-36 (2019)
Article DOI: 10.1016/j.ejmech.2018.11.039
BindingDB Entry DOI: 10.7270/Q2930XFF |
More data for this
Ligand-Target Pair | |
Cytochrome P450 1B1
(Homo sapiens (Human)) | BDBM50507597
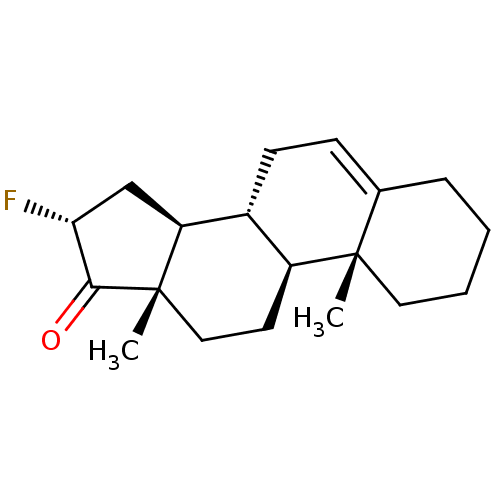
(CHEMBL4441355)Show SMILES [H][C@@]12C[C@@H](F)C(=O)[C@@]1(C)CC[C@@]1([H])[C@@]2([H])CC=C2CCCC[C@]12C |r,t:18| Show InChI InChI=1S/C19H27FO/c1-18-9-4-3-5-12(18)6-7-13-14(18)8-10-19(2)15(13)11-16(20)17(19)21/h6,13-16H,3-5,7-11H2,1-2H3/t13-,14+,15+,16-,18+,19+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 500 | n/a | n/a | n/a | n/a | n/a | n/a |
Birla Institute of Technology
Curated by ChEMBL
| Assay Description
Inhibition of CYP1B1 in human MCF7 cells assessed as reduction in DMBA-induced CYP1B1-mediated EROD activity by EROD assay |
Eur J Med Chem 163: 28-36 (2019)
Article DOI: 10.1016/j.ejmech.2018.11.039
BindingDB Entry DOI: 10.7270/Q2930XFF |
More data for this
Ligand-Target Pair | |
Cytochrome P450 1A2
(Homo sapiens (Human)) | BDBM50507596

(CHEMBL4532099)Show SMILES COc1cccc2c(OC)cc3c([nH]c(cc3=O)-c3cccc(Br)c3)c12 Show InChI InChI=1S/C21H16BrNO3/c1-25-18-8-4-7-14-19(26-2)10-15-17(24)11-16(23-21(15)20(14)18)12-5-3-6-13(22)9-12/h3-11H,1-2H3,(H,23,24) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 588 | n/a | n/a | n/a | n/a | n/a | n/a |
Birla Institute of Technology
Curated by ChEMBL
| Assay Description
Inhibition of human CYP1A2 using 7-Ethoxyresorufin as substrate after 50 mins in presence of NADP+ by EROD assay |
Eur J Med Chem 163: 28-36 (2019)
Article DOI: 10.1016/j.ejmech.2018.11.039
BindingDB Entry DOI: 10.7270/Q2930XFF |
More data for this
Ligand-Target Pair | |
Cytochrome P450 1B1
(Homo sapiens (Human)) | BDBM23926

((E)-resveratrol | 5-[(E)-2-(4-hydroxyphenyl)etheny...)Show InChI InChI=1S/C14H12O3/c15-12-5-3-10(4-6-12)1-2-11-7-13(16)9-14(17)8-11/h1-9,15-17H/b2-1+ | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 1.40E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Birla Institute of Technology
Curated by ChEMBL
| Assay Description
Inhibition of human CYP1B1 by EROD assay |
Eur J Med Chem 163: 28-36 (2019)
Article DOI: 10.1016/j.ejmech.2018.11.039
BindingDB Entry DOI: 10.7270/Q2930XFF |
More data for this
Ligand-Target Pair | |
Cytochrome P450 1B1
(Homo sapiens (Human)) | BDBM50507597

(CHEMBL4441355)Show SMILES [H][C@@]12C[C@@H](F)C(=O)[C@@]1(C)CC[C@@]1([H])[C@@]2([H])CC=C2CCCC[C@]12C |r,t:18| Show InChI InChI=1S/C19H27FO/c1-18-9-4-3-5-12(18)6-7-13-14(18)8-10-19(2)15(13)11-16(20)17(19)21/h6,13-16H,3-5,7-11H2,1-2H3/t13-,14+,15+,16-,18+,19+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 2.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Birla Institute of Technology
Curated by ChEMBL
| Assay Description
Inhibition of CYP1B1 in human MCF7 cells assessed as reduction in TCDD-induced CYP1B1-mediated EROD activity by EROD assay |
Eur J Med Chem 163: 28-36 (2019)
Article DOI: 10.1016/j.ejmech.2018.11.039
BindingDB Entry DOI: 10.7270/Q2930XFF |
More data for this
Ligand-Target Pair | |
Cytochrome P450 1B1
(Homo sapiens (Human)) | BDBM50203126

(3,4-dimethoxy-6,7-dihydro-[1,3]dioxolo[4,5-g]pyrid...)Show InChI InChI=1S/C20H18NO4/c1-22-17-4-3-12-7-16-14-9-19-18(24-11-25-19)8-13(14)5-6-21(16)10-15(12)20(17)23-2/h3-4,7-10H,5-6,11H2,1-2H3/q+1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
PC cid
PC sid
PDB
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Birla Institute of Technology
Curated by ChEMBL
| Assay Description
Inhibition of CYP1B1 in human HepG2 cells |
Eur J Med Chem 163: 28-36 (2019)
Article DOI: 10.1016/j.ejmech.2018.11.039
BindingDB Entry DOI: 10.7270/Q2930XFF |
More data for this
Ligand-Target Pair | |
Cytochrome P450 1A2
(Homo sapiens (Human)) | BDBM50507599

(CHEMBL4456952)Show SMILES COc1ccc(cc1)-c1cc(=O)c2cc(OC)c3cccc(OC)c3c2[nH]1 Show InChI InChI=1S/C22H19NO4/c1-25-14-9-7-13(8-10-14)17-12-18(24)16-11-20(27-3)15-5-4-6-19(26-2)21(15)22(16)23-17/h4-12H,1-3H3,(H,23,24) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | >5.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Birla Institute of Technology
Curated by ChEMBL
| Assay Description
Inhibition of human CYP1A2 using 7-Ethoxyresorufin as substrate after 50 mins in presence of NADP+ by EROD assay |
Eur J Med Chem 163: 28-36 (2019)
Article DOI: 10.1016/j.ejmech.2018.11.039
BindingDB Entry DOI: 10.7270/Q2930XFF |
More data for this
Ligand-Target Pair | |
Cytochrome P450 1A1
(Homo sapiens (Human)) | BDBM23926

((E)-resveratrol | 5-[(E)-2-(4-hydroxyphenyl)etheny...)Show InChI InChI=1S/C14H12O3/c15-12-5-3-10(4-6-12)1-2-11-7-13(16)9-14(17)8-11/h1-9,15-17H/b2-1+ | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 1.10E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Birla Institute of Technology
Curated by ChEMBL
| Assay Description
Inhibition of human CYP1A1 using ethoxyresorufin as substrate by MROD assay |
Eur J Med Chem 163: 28-36 (2019)
Article DOI: 10.1016/j.ejmech.2018.11.039
BindingDB Entry DOI: 10.7270/Q2930XFF |
More data for this
Ligand-Target Pair | |
Cytochrome P450 1A1
(Homo sapiens (Human)) | BDBM23926

((E)-resveratrol | 5-[(E)-2-(4-hydroxyphenyl)etheny...)Show InChI InChI=1S/C14H12O3/c15-12-5-3-10(4-6-12)1-2-11-7-13(16)9-14(17)8-11/h1-9,15-17H/b2-1+ | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 2.30E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Birla Institute of Technology
Curated by ChEMBL
| Assay Description
Inhibition of human CYP1A1 using ethoxyresorufin as substrate by EROD assay |
Eur J Med Chem 163: 28-36 (2019)
Article DOI: 10.1016/j.ejmech.2018.11.039
BindingDB Entry DOI: 10.7270/Q2930XFF |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data