 Found 52 hits Enz. Inhib. hit(s) with all data for entry = 50008459
Found 52 hits Enz. Inhib. hit(s) with all data for entry = 50008459 Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Adenosine deaminase
(Plasmodium falciparum) | BDBM50519495

(CHEMBL34023)Show SMILES OC[C@H]1O[C@H]([C@H](O)[C@@H]1O)n1cnc2[C@@H](O)CN=CNc12 |c:17| Show InChI InChI=1S/C11H16N4O5/c16-2-6-8(18)9(19)11(20-6)15-4-14-7-5(17)1-12-3-13-10(7)15/h3-6,8-9,11,16-19H,1-2H2,(H,12,13)/t5-,6+,8+,9+,11+/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/TrEMBL
DrugBank
GoogleScholar
AffyNet 
| Purchase
PC cid
PC sid
UniChem
| Article
PubMed
| 0.00700 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ Montpellier
Curated by ChEMBL
| Assay Description
Inhibition of Plasmodium falciparum ADA assessed as reduction in formation of ammonia using adenosine as substrate incubated for 15 mins by spectroph... |
J Med Chem 62: 8365-8391 (2019)
Article DOI: 10.1021/acs.jmedchem.9b00182
BindingDB Entry DOI: 10.7270/Q2T1571N |
More data for this
Ligand-Target Pair | |
Purine nucleoside phosphorylase
(Homo sapiens (Human)) | BDBM50293087
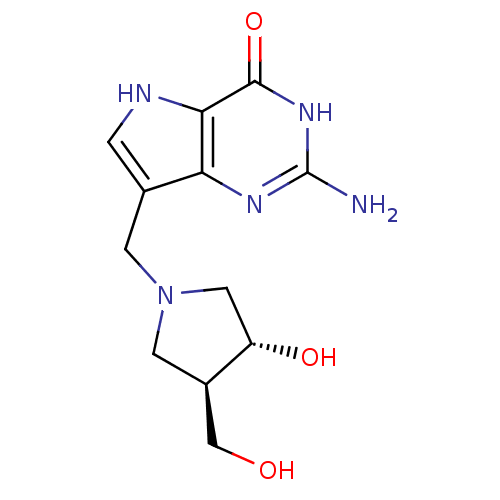
(2-amino-7-(((3R,4R)-3-hydroxy-4-(hydroxymethyl)pyr...)Show SMILES Nc1nc2c(CN3C[C@H](O)[C@@H](CO)C3)c[nH]c2c(=O)[nH]1 |r| Show InChI InChI=1S/C12H17N5O3/c13-12-15-9-6(1-14-10(9)11(20)16-12)2-17-3-7(5-18)8(19)4-17/h1,7-8,14,18-19H,2-5H2,(H3,13,15,16,20)/t7-,8+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
MMDB
PC cid
PC sid
PDB
UniChem
Similars
| PDB
Article
PubMed
| 0.00700 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ Montpellier
Curated by ChEMBL
| Assay Description
Inhibition of human His-tagged PNP assessed as equilibrium dissociation constant by measuring reduction in uric acid formation by spectrophotometric ... |
J Med Chem 62: 8365-8391 (2019)
Article DOI: 10.1021/acs.jmedchem.9b00182
BindingDB Entry DOI: 10.7270/Q2T1571N |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Purine nucleoside phosphorylase
(Homo sapiens (Human)) | BDBM22109
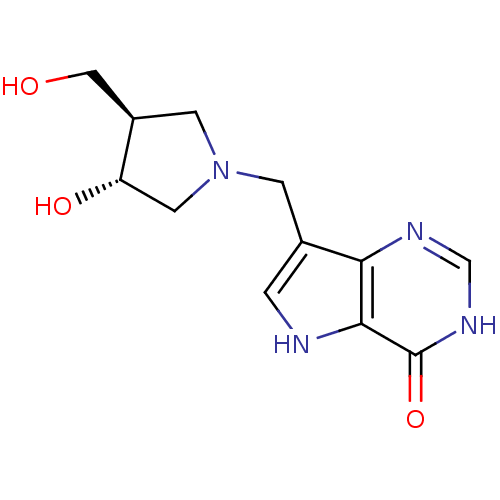
(7-{[(3R,4R)-3-hydroxy-4-(hydroxymethyl)pyrrolidin-...)Show SMILES OC[C@H]1CN(Cc2c[nH]c3c2nc[nH]c3=O)C[C@@H]1O |r| Show InChI InChI=1S/C12H16N4O3/c17-5-8-3-16(4-9(8)18)2-7-1-13-11-10(7)14-6-15-12(11)19/h1,6,8-9,13,17-18H,2-5H2,(H,14,15,19)/t8-,9+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
MMDB
PC cid
PC sid
PDB
UniChem
Similars
| MMDB
PDB
Article
PubMed
| 0.0160 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ Montpellier
Curated by ChEMBL
| Assay Description
Inhibition of human His-tagged PNP assessed as equilibrium dissociation constant by measuring reduction in uric acid formation by spectrophotometric ... |
J Med Chem 62: 8365-8391 (2019)
Article DOI: 10.1021/acs.jmedchem.9b00182
BindingDB Entry DOI: 10.7270/Q2T1571N |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Adenosine deaminase
(Homo sapiens (Human)) | BDBM22925

((8R)-3-[(2R,4S,5R)-4-hydroxy-5-(hydroxymethyl)oxol...)Show SMILES OC[C@H]1O[C@H](C[C@@H]1O)n1cnc2[C@H](O)CNC=Nc12 |c:17| Show InChI InChI=1S/C11H16N4O4/c16-3-8-6(17)1-9(19-8)15-5-14-10-7(18)2-12-4-13-11(10)15/h4-9,16-18H,1-3H2,(H,12,13)/t6-,7+,8+,9+/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
DrugBank
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| DrugBank
Article
PubMed
| 0.0260 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ Montpellier
Curated by ChEMBL
| Assay Description
Inhibition of human erythrocytes ADA assessed as equilibrium dissociation constant by measuring reduction in formation of inosine using adenosine as ... |
J Med Chem 62: 8365-8391 (2019)
Article DOI: 10.1021/acs.jmedchem.9b00182
BindingDB Entry DOI: 10.7270/Q2T1571N |
More data for this
Ligand-Target Pair | |
Adenosine deaminase
(Plasmodium falciparum) | BDBM22925

((8R)-3-[(2R,4S,5R)-4-hydroxy-5-(hydroxymethyl)oxol...)Show SMILES OC[C@H]1O[C@H](C[C@@H]1O)n1cnc2[C@H](O)CNC=Nc12 |c:17| Show InChI InChI=1S/C11H16N4O4/c16-3-8-6(17)1-9(19-8)15-5-14-10-7(18)2-12-4-13-11(10)15/h4-9,16-18H,1-3H2,(H,12,13)/t6-,7+,8+,9+/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/TrEMBL
DrugBank
GoogleScholar
AffyNet 
| Purchase
DrugBank
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| 0.0380 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ Montpellier
Curated by ChEMBL
| Assay Description
Inhibition of Plasmodium falciparum N-terminal thrombin cleavable His6-tagged ADA expressed in Escherichia coli BL21 assessed as equilibrium dissocia... |
J Med Chem 62: 8365-8391 (2019)
Article DOI: 10.1021/acs.jmedchem.9b00182
BindingDB Entry DOI: 10.7270/Q2T1571N |
More data for this
Ligand-Target Pair | |
Adenosine deaminase
(Plasmodium falciparum) | BDBM50367032

(COFORMYCIN)Show SMILES OC[C@H]1O[C@H]([C@H](O)[C@@H]1O)n1cnc2[C@H](O)CNC=Nc12 |r,c:18| Show InChI InChI=1S/C11H16N4O5/c16-2-6-8(18)9(19)11(20-6)15-4-14-7-5(17)1-12-3-13-10(7)15/h3-6,8-9,11,16-19H,1-2H2,(H,12,13)/t5-,6-,8-,9-,11-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/TrEMBL
DrugBank
GoogleScholar
AffyNet 
| KEGG
PC cid
PC sid
PDB
UniChem
Similars
| Article
PubMed
| 0.0800 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ Montpellier
Curated by ChEMBL
| Assay Description
Inhibition of Plasmodium falciparum N-terminal thrombin cleavable His6-tagged ADA expressed in Escherichia coli BL21 assessed as equilibrium dissocia... |
J Med Chem 62: 8365-8391 (2019)
Article DOI: 10.1021/acs.jmedchem.9b00182
BindingDB Entry DOI: 10.7270/Q2T1571N |
More data for this
Ligand-Target Pair | |
Adenosine deaminase
(Homo sapiens (Human)) | BDBM50367032

(COFORMYCIN)Show SMILES OC[C@H]1O[C@H]([C@H](O)[C@@H]1O)n1cnc2[C@H](O)CNC=Nc12 |r,c:18| Show InChI InChI=1S/C11H16N4O5/c16-2-6-8(18)9(19)11(20-6)15-4-14-7-5(17)1-12-3-13-10(7)15/h3-6,8-9,11,16-19H,1-2H2,(H,12,13)/t5-,6-,8-,9-,11-/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| KEGG
PC cid
PC sid
PDB
UniChem
Similars
| Article
PubMed
| 0.110 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ Montpellier
Curated by ChEMBL
| Assay Description
Inhibition of human erythrocytes ADA assessed as equilibrium dissociation constant by measuring reduction in formation of inosine using adenosine as ... |
J Med Chem 62: 8365-8391 (2019)
Article DOI: 10.1021/acs.jmedchem.9b00182
BindingDB Entry DOI: 10.7270/Q2T1571N |
More data for this
Ligand-Target Pair | |
Purine nucleoside phosphorylase
(Homo sapiens (Human)) | BDBM50293087

(2-amino-7-(((3R,4R)-3-hydroxy-4-(hydroxymethyl)pyr...)Show SMILES Nc1nc2c(CN3C[C@H](O)[C@@H](CO)C3)c[nH]c2c(=O)[nH]1 |r| Show InChI InChI=1S/C12H17N5O3/c13-12-15-9-6(1-14-10(9)11(20)16-12)2-17-3-7(5-18)8(19)4-17/h1,7-8,14,18-19H,2-5H2,(H3,13,15,16,20)/t7-,8+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
MMDB
PC cid
PC sid
PDB
UniChem
Similars
| PDB
Article
PubMed
| 0.160 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ Montpellier
Curated by ChEMBL
| Assay Description
Inhibition of human His-tagged PNP assessed as inhibitor constant for enzyme-inhibitor complex formation by measuring reduction in uric acid formatio... |
J Med Chem 62: 8365-8391 (2019)
Article DOI: 10.1021/acs.jmedchem.9b00182
BindingDB Entry DOI: 10.7270/Q2T1571N |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Adenosine deaminase
(Plasmodium falciparum) | BDBM50367032

(COFORMYCIN)Show SMILES OC[C@H]1O[C@H]([C@H](O)[C@@H]1O)n1cnc2[C@H](O)CNC=Nc12 |r,c:18| Show InChI InChI=1S/C11H16N4O5/c16-2-6-8(18)9(19)11(20-6)15-4-14-7-5(17)1-12-3-13-10(7)15/h3-6,8-9,11,16-19H,1-2H2,(H,12,13)/t5-,6-,8-,9-,11-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/TrEMBL
DrugBank
GoogleScholar
AffyNet 
| KEGG
PC cid
PC sid
PDB
UniChem
Similars
| Article
PubMed
| 0.25 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ Montpellier
Curated by ChEMBL
| Assay Description
Inhibition of Plasmodium falciparum ADA assessed as reduction in formation of ammonia using adenosine as substrate incubated for 15 mins by spectroph... |
J Med Chem 62: 8365-8391 (2019)
Article DOI: 10.1021/acs.jmedchem.9b00182
BindingDB Entry DOI: 10.7270/Q2T1571N |
More data for this
Ligand-Target Pair | |
Adenosine deaminase
(Plasmodium falciparum) | BDBM50519494

(CHEMBL1234234)Show SMILES CSC[C@H]1O[C@H]([C@H](O)[C@@H]1O)n1cnc2[C@H](O)CNC=Nc12 |r,c:19| Show InChI InChI=1S/C12H18N4O4S/c1-21-3-7-9(18)10(19)12(20-7)16-5-15-8-6(17)2-13-4-14-11(8)16/h4-7,9-10,12,17-19H,2-3H2,1H3,(H,13,14)/t6-,7-,9-,10-,12-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/TrEMBL
DrugBank
GoogleScholar
AffyNet 
| PC cid
PC sid
PDB
UniChem
| Article
PubMed
| 0.430 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ Montpellier
Curated by ChEMBL
| Assay Description
Inhibition of Plasmodium falciparum N-terminal thrombin cleavable His6-tagged ADA expressed in Escherichia coli BL21 assessed as equilibrium dissocia... |
J Med Chem 62: 8365-8391 (2019)
Article DOI: 10.1021/acs.jmedchem.9b00182
BindingDB Entry DOI: 10.7270/Q2T1571N |
More data for this
Ligand-Target Pair | |
Purine nucleoside phosphorylase
(Plasmodium falciparum) | BDBM22109

(7-{[(3R,4R)-3-hydroxy-4-(hydroxymethyl)pyrrolidin-...)Show SMILES OC[C@H]1CN(Cc2c[nH]c3c2nc[nH]c3=O)C[C@@H]1O |r| Show InChI InChI=1S/C12H16N4O3/c17-5-8-3-16(4-9(8)18)2-7-1-13-11-10(7)14-6-15-12(11)19/h1,6,8-9,13,17-18H,2-5H2,(H,14,15,19)/t8-,9+/m1/s1 | PDB
MMDB
KEGG
UniProtKB/TrEMBL
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
MMDB
PC cid
PC sid
PDB
UniChem
Similars
| Article
PubMed
| 0.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ Montpellier
Curated by ChEMBL
| Assay Description
Inhibition of Plasmodium falciparum His-tagged PNP expressed in Escherichia coli assessed as inhibitor constant for enzyme-inhibitor complex formatio... |
J Med Chem 62: 8365-8391 (2019)
Article DOI: 10.1021/acs.jmedchem.9b00182
BindingDB Entry DOI: 10.7270/Q2T1571N |
More data for this
Ligand-Target Pair | |
Adenosine deaminase
(Homo sapiens (Human)) | BDBM22925

((8R)-3-[(2R,4S,5R)-4-hydroxy-5-(hydroxymethyl)oxol...)Show SMILES OC[C@H]1O[C@H](C[C@@H]1O)n1cnc2[C@H](O)CNC=Nc12 |c:17| Show InChI InChI=1S/C11H16N4O4/c16-3-8-6(17)1-9(19-8)15-5-14-10-7(18)2-12-4-13-11(10)15/h4-9,16-18H,1-3H2,(H,12,13)/t6-,7+,8+,9+/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
DrugBank
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| DrugBank
Article
PubMed
| 0.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ Montpellier
Curated by ChEMBL
| Assay Description
Inhibition of human erythrocytes ADA assessed as reduction in formation of inosine using adenosine as substrate |
J Med Chem 62: 8365-8391 (2019)
Article DOI: 10.1021/acs.jmedchem.9b00182
BindingDB Entry DOI: 10.7270/Q2T1571N |
More data for this
Ligand-Target Pair | |
Purine nucleoside phosphorylase
(Plasmodium falciparum) | BDBM50195587

(1,4-DIDEOXY-4-AZA-1-(S)-(9-DEAZAHYPOXANTHIN-9-YL)-...)Show SMILES OC[C@H]1N[C@H]([C@H](O)[C@@H]1O)c1c[nH]c2c1nc[nH]c2=O |r| Show InChI InChI=1S/C11H14N4O4/c16-2-5-9(17)10(18)7(15-5)4-1-12-8-6(4)13-3-14-11(8)19/h1,3,5,7,9-10,12,15-18H,2H2,(H,13,14,19)/t5-,7+,9-,10+/m1/s1 | PDB
MMDB
KEGG
UniProtKB/TrEMBL
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
Article
PubMed
| 0.600 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ Montpellier
Curated by ChEMBL
| Assay Description
Inhibition of Plasmodium falciparum His-tagged PNP assessed as inhibitor constant for enzyme-inhibitor-substrate complex formation |
J Med Chem 62: 8365-8391 (2019)
Article DOI: 10.1021/acs.jmedchem.9b00182
BindingDB Entry DOI: 10.7270/Q2T1571N |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Purine nucleoside phosphorylase
(Plasmodium falciparum) | BDBM50247158

(7-(((3S,4S)-3-hydroxy-4-(hydroxymethyl)pyrrolidin-...)Show SMILES OC[C@@H]1CN(Cc2c[nH]c3c2nc[nH]c3=O)C[C@H]1O |r| Show InChI InChI=1S/C12H16N4O3/c17-5-8-3-16(4-9(8)18)2-7-1-13-11-10(7)14-6-15-12(11)19/h1,6,8-9,13,17-18H,2-5H2,(H,14,15,19)/t8-,9+/m0/s1 | PDB
MMDB
KEGG
UniProtKB/TrEMBL
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
MMDB
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.680 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ Montpellier
Curated by ChEMBL
| Assay Description
Inhibition of Plasmodium falciparum His-tagged PNP assessed as inhibitor constant for enzyme-inhibitor-substrate complex formation |
J Med Chem 62: 8365-8391 (2019)
Article DOI: 10.1021/acs.jmedchem.9b00182
BindingDB Entry DOI: 10.7270/Q2T1571N |
More data for this
Ligand-Target Pair | |
Adenosine deaminase
(Plasmodium falciparum) | BDBM50367032

(COFORMYCIN)Show SMILES OC[C@H]1O[C@H]([C@H](O)[C@@H]1O)n1cnc2[C@H](O)CNC=Nc12 |r,c:18| Show InChI InChI=1S/C11H16N4O5/c16-2-6-8(18)9(19)11(20-6)15-4-14-7-5(17)1-12-3-13-10(7)15/h3-6,8-9,11,16-19H,1-2H2,(H,12,13)/t5-,6-,8-,9-,11-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/TrEMBL
DrugBank
GoogleScholar
AffyNet 
| KEGG
PC cid
PC sid
PDB
UniChem
Similars
| Article
PubMed
| 0.680 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ Montpellier
Curated by ChEMBL
| Assay Description
Inhibition of Plasmodium falciparum N-terminal thrombin cleavable His6-tagged ADA expressed in Escherichia coli BL21 assessed as reduction in formati... |
J Med Chem 62: 8365-8391 (2019)
Article DOI: 10.1021/acs.jmedchem.9b00182
BindingDB Entry DOI: 10.7270/Q2T1571N |
More data for this
Ligand-Target Pair | |
Purine nucleoside phosphorylase
(Plasmodium falciparum) | BDBM50293087

(2-amino-7-(((3R,4R)-3-hydroxy-4-(hydroxymethyl)pyr...)Show SMILES Nc1nc2c(CN3C[C@H](O)[C@@H](CO)C3)c[nH]c2c(=O)[nH]1 |r| Show InChI InChI=1S/C12H17N5O3/c13-12-15-9-6(1-14-10(9)11(20)16-12)2-17-3-7(5-18)8(19)4-17/h1,7-8,14,18-19H,2-5H2,(H3,13,15,16,20)/t7-,8+/m1/s1 | PDB
MMDB
KEGG
UniProtKB/TrEMBL
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
MMDB
PC cid
PC sid
PDB
UniChem
Similars
| PDB
Article
PubMed
| 0.890 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ Montpellier
Curated by ChEMBL
| Assay Description
Inhibition of Plasmodium falciparum His-tagged PNP expressed in Escherichia coli assessed as inhibitor constant for enzyme-inhibitor complex formatio... |
J Med Chem 62: 8365-8391 (2019)
Article DOI: 10.1021/acs.jmedchem.9b00182
BindingDB Entry DOI: 10.7270/Q2T1571N |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Purine nucleoside phosphorylase
(Plasmodium falciparum) | BDBM50293088
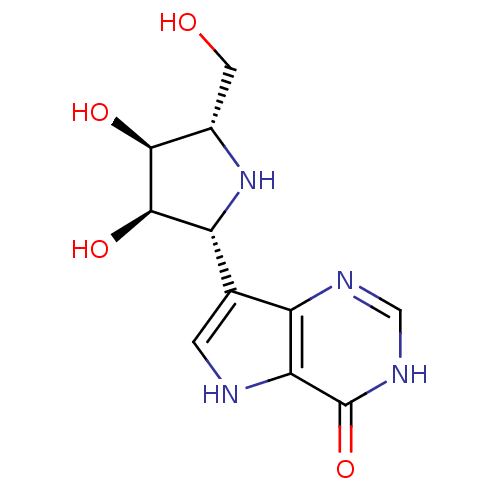
(7-((2R,3R,4S,5S)-3,4-dihydroxy-5-(hydroxymethyl)py...)Show SMILES OC[C@@H]1N[C@@H]([C@@H](O)[C@H]1O)c1c[nH]c2c1nc[nH]c2=O |r| Show InChI InChI=1S/C11H14N4O4/c16-2-5-9(17)10(18)7(15-5)4-1-12-8-6(4)13-3-14-11(8)19/h1,3,5,7,9-10,12,15-18H,2H2,(H,13,14,19)/t5-,7+,9-,10+/m0/s1 | PDB
MMDB
KEGG
UniProtKB/TrEMBL
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
MMDB
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.900 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ Montpellier
Curated by ChEMBL
| Assay Description
Inhibition of Plasmodium falciparum His-tagged PNP assessed as inhibitor constant for enzyme-inhibitor-substrate complex formation |
J Med Chem 62: 8365-8391 (2019)
Article DOI: 10.1021/acs.jmedchem.9b00182
BindingDB Entry DOI: 10.7270/Q2T1571N |
More data for this
Ligand-Target Pair | |
Purine nucleoside phosphorylase
(Plasmodium falciparum) | BDBM50293086
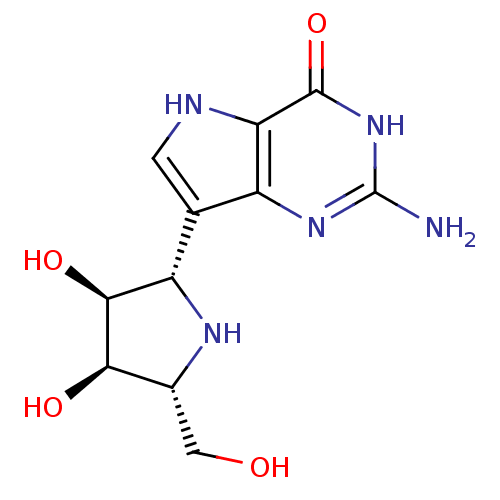
((1S)-1,4-dideoxy-1,4-imino-1-(9-deazaguanin-9-yl)-...)Show SMILES Nc1nc2c(c[nH]c2c(=O)[nH]1)[C@@H]1N[C@H](CO)[C@@H](O)[C@H]1O |r| Show InChI InChI=1S/C11H15N5O4/c12-11-15-5-3(1-13-7(5)10(20)16-11)6-9(19)8(18)4(2-17)14-6/h1,4,6,8-9,13-14,17-19H,2H2,(H3,12,15,16,20)/t4-,6+,8-,9+/m1/s1 | PDB
MMDB
KEGG
UniProtKB/TrEMBL
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| 0.900 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ Montpellier
Curated by ChEMBL
| Assay Description
Inhibition of Plasmodium falciparum His-tagged PNP assessed as inhibitor constant for enzyme-inhibitor-substrate complex formation |
J Med Chem 62: 8365-8391 (2019)
Article DOI: 10.1021/acs.jmedchem.9b00182
BindingDB Entry DOI: 10.7270/Q2T1571N |
More data for this
Ligand-Target Pair | |
Purine nucleoside phosphorylase
(Homo sapiens (Human)) | BDBM22109

(7-{[(3R,4R)-3-hydroxy-4-(hydroxymethyl)pyrrolidin-...)Show SMILES OC[C@H]1CN(Cc2c[nH]c3c2nc[nH]c3=O)C[C@@H]1O |r| Show InChI InChI=1S/C12H16N4O3/c17-5-8-3-16(4-9(8)18)2-7-1-13-11-10(7)14-6-15-12(11)19/h1,6,8-9,13,17-18H,2-5H2,(H,14,15,19)/t8-,9+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
MMDB
PC cid
PC sid
PDB
UniChem
Similars
| MMDB
PDB
Article
PubMed
| 1.10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ Montpellier
Curated by ChEMBL
| Assay Description
Inhibition of human His-tagged PNP assessed as inhibitor constant for enzyme-inhibitor complex formation by measuring reduction in uric acid formatio... |
J Med Chem 62: 8365-8391 (2019)
Article DOI: 10.1021/acs.jmedchem.9b00182
BindingDB Entry DOI: 10.7270/Q2T1571N |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Hypoxanthine-guanine phosphoribosyltransferase
(Homo sapiens (Human)) | BDBM50467705
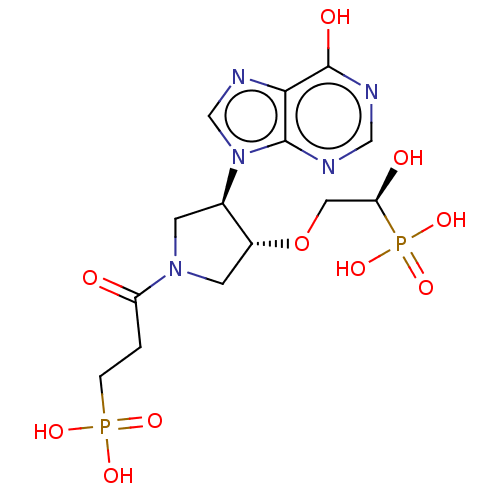
(CHEMBL4282830)Show SMILES O[C@H](CO[C@@H]1CN(C[C@H]1n1cnc2c(O)ncnc12)C(=O)CCP(O)(O)=O)P(O)(O)=O |r| Show InChI InChI=1S/C14H21N5O10P2/c20-10(1-2-30(23,24)25)18-3-8(9(4-18)29-5-11(21)31(26,27)28)19-7-17-12-13(19)15-6-16-14(12)22/h6-9,11,21H,1-5H2,(H,15,16,22)(H2,23,24,25)(H2,26,27,28)/t8-,9-,11+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 1.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ Montpellier
Curated by ChEMBL
| Assay Description
Inhibition of human HGPRT using Prib-PP as substrate by Hanes-plot based method |
J Med Chem 62: 8365-8391 (2019)
Article DOI: 10.1021/acs.jmedchem.9b00182
BindingDB Entry DOI: 10.7270/Q2T1571N |
More data for this
Ligand-Target Pair | |
Purine nucleoside phosphorylase
(Plasmodium falciparum) | BDBM50247158

(7-(((3S,4S)-3-hydroxy-4-(hydroxymethyl)pyrrolidin-...)Show SMILES OC[C@@H]1CN(Cc2c[nH]c3c2nc[nH]c3=O)C[C@H]1O |r| Show InChI InChI=1S/C12H16N4O3/c17-5-8-3-16(4-9(8)18)2-7-1-13-11-10(7)14-6-15-12(11)19/h1,6,8-9,13,17-18H,2-5H2,(H,14,15,19)/t8-,9+/m0/s1 | PDB
MMDB
KEGG
UniProtKB/TrEMBL
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
MMDB
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ Montpellier
Curated by ChEMBL
| Assay Description
Inhibition of Plasmodium falciparum His-tagged PNP assessed as inhibitor constant for enzyme-inhibitor complex formation |
J Med Chem 62: 8365-8391 (2019)
Article DOI: 10.1021/acs.jmedchem.9b00182
BindingDB Entry DOI: 10.7270/Q2T1571N |
More data for this
Ligand-Target Pair | |
Hypoxanthine-guanine phosphoribosyltransferase
(Homo sapiens (Human)) | BDBM50519492
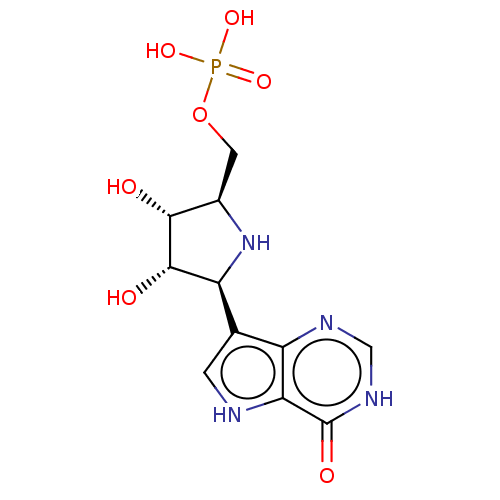
(CHEMBL1233663)Show SMILES O[C@@H]1[C@@H](COP(O)(O)=O)N[C@H]([C@@H]1O)c1c[nH]c2c1nc[nH]c2=O |r| Show InChI InChI=1S/C11H15N4O7P/c16-9-5(2-22-23(19,20)21)15-7(10(9)17)4-1-12-8-6(4)13-3-14-11(8)18/h1,3,5,7,9-10,12,15-17H,2H2,(H,13,14,18)(H2,19,20,21)/t5-,7+,9-,10+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
PDB
UniChem
| Article
PubMed
| 1.80 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ Montpellier
Curated by ChEMBL
| Assay Description
Inhibition of human HGPRT assessed as inhibitor constant for enzyme-inhibitor-substrate complex formation using [5'-14C]IMP as substrate by scintilla... |
J Med Chem 62: 8365-8391 (2019)
Article DOI: 10.1021/acs.jmedchem.9b00182
BindingDB Entry DOI: 10.7270/Q2T1571N |
More data for this
Ligand-Target Pair | |
Adenosine deaminase
(Plasmodium falciparum) | BDBM50519494

(CHEMBL1234234)Show SMILES CSC[C@H]1O[C@H]([C@H](O)[C@@H]1O)n1cnc2[C@H](O)CNC=Nc12 |r,c:19| Show InChI InChI=1S/C12H18N4O4S/c1-21-3-7-9(18)10(19)12(20-7)16-5-15-8-6(17)2-13-4-14-11(8)16/h4-7,9-10,12,17-19H,2-3H2,1H3,(H,13,14)/t6-,7-,9-,10-,12-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/TrEMBL
DrugBank
GoogleScholar
AffyNet 
| PC cid
PC sid
PDB
UniChem
| Article
PubMed
| 2.70 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ Montpellier
Curated by ChEMBL
| Assay Description
Inhibition of Plasmodium falciparum N-terminal thrombin cleavable His6-tagged ADA expressed in Escherichia coli BL21 assessed as reduction in formati... |
J Med Chem 62: 8365-8391 (2019)
Article DOI: 10.1021/acs.jmedchem.9b00182
BindingDB Entry DOI: 10.7270/Q2T1571N |
More data for this
Ligand-Target Pair | |
Purine nucleoside phosphorylase
(Plasmodium falciparum) | BDBM50247149
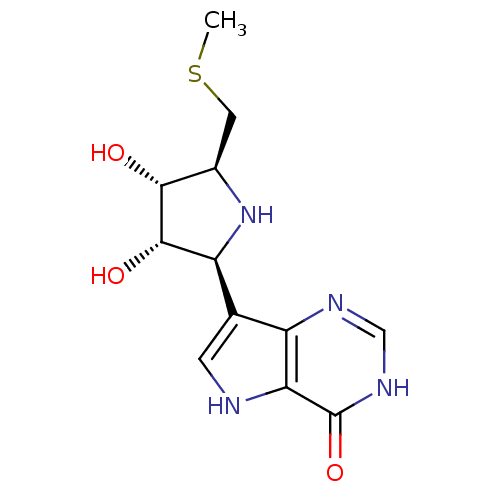
(5'-Methylthio-ImmH | CHEMBL473929 | US9290501, (A))Show SMILES CSC[C@H]1N[C@H]([C@H](O)[C@@H]1O)c1c[nH]c2c1nc[nH]c2=O |r| Show InChI InChI=1S/C12H16N4O3S/c1-20-3-6-10(17)11(18)8(16-6)5-2-13-9-7(5)14-4-15-12(9)19/h2,4,6,8,10-11,13,16-18H,3H2,1H3,(H,14,15,19)/t6-,8+,10-,11+/m1/s1 | PDB
MMDB
KEGG
UniProtKB/TrEMBL
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
MMDB
PC cid
PC sid
UniChem
Similars
| MMDB
Article
PubMed
| 2.70 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ Montpellier
Curated by ChEMBL
| Assay Description
Inhibition of Plasmodium falciparum 3D7 PNP expressed in Escherichia coli assessed as reduction in uric acid formation by spectrophotometric method |
J Med Chem 62: 8365-8391 (2019)
Article DOI: 10.1021/acs.jmedchem.9b00182
BindingDB Entry DOI: 10.7270/Q2T1571N |
More data for this
Ligand-Target Pair | |
Hypoxanthine-guanine phosphoribosyltransferase
(Homo sapiens (Human)) | BDBM50467710
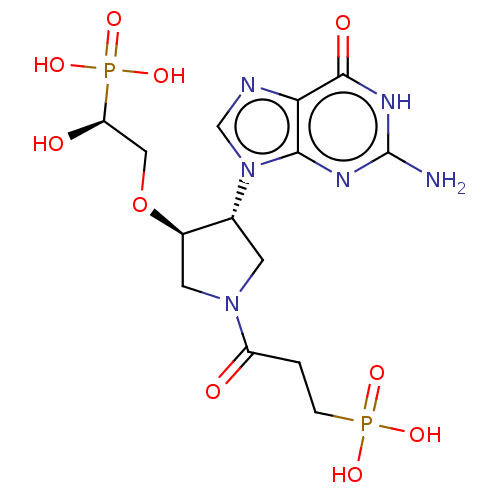
(CHEMBL4290716)Show SMILES Nc1nc2n(cnc2c(=O)[nH]1)[C@@H]1CN(C[C@H]1OC[C@@H](O)P(O)(O)=O)C(=O)CCP(O)(O)=O |r| Show InChI InChI=1S/C14H22N6O10P2/c15-14-17-12-11(13(23)18-14)16-6-20(12)7-3-19(9(21)1-2-31(24,25)26)4-8(7)30-5-10(22)32(27,28)29/h6-8,10,22H,1-5H2,(H2,24,25,26)(H2,27,28,29)(H3,15,17,18,23)/t7-,8-,10+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
PDB
UniChem
| PDB
Article
PubMed
| 3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ Montpellier
Curated by ChEMBL
| Assay Description
Inhibition of human HGPRT using Prib-PP as substrate by Hanes-plot based method |
J Med Chem 62: 8365-8391 (2019)
Article DOI: 10.1021/acs.jmedchem.9b00182
BindingDB Entry DOI: 10.7270/Q2T1571N |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Hypoxanthine-guanine phosphoribosyltransferase
(Homo sapiens (Human)) | BDBM50519500

(CHEMBL1233603)Show SMILES Nc1nc2c(c[nH]c2c(=O)[nH]1)[C@@H]1N[C@H](COP(O)(O)=O)[C@@H](O)[C@H]1O |r| Show InChI InChI=1S/C11H16N5O7P/c12-11-15-5-3(1-13-7(5)10(19)16-11)6-9(18)8(17)4(14-6)2-23-24(20,21)22/h1,4,6,8-9,13-14,17-18H,2H2,(H2,20,21,22)(H3,12,15,16,19)/t4-,6+,8-,9+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
PDB
UniChem
| PDB
Article
PubMed
| 4.60 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ Montpellier
Curated by ChEMBL
| Assay Description
Inhibition of human HGPRT assessed as inhibitor constant for enzyme-inhibitor-substrate complex formation using [5'-14C]IMP as substrate by scintilla... |
J Med Chem 62: 8365-8391 (2019)
Article DOI: 10.1021/acs.jmedchem.9b00182
BindingDB Entry DOI: 10.7270/Q2T1571N |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Adenosine deaminase
(Plasmodium falciparum) | BDBM22925

((8R)-3-[(2R,4S,5R)-4-hydroxy-5-(hydroxymethyl)oxol...)Show SMILES OC[C@H]1O[C@H](C[C@@H]1O)n1cnc2[C@H](O)CNC=Nc12 |c:17| Show InChI InChI=1S/C11H16N4O4/c16-3-8-6(17)1-9(19-8)15-5-14-10-7(18)2-12-4-13-11(10)15/h4-9,16-18H,1-3H2,(H,12,13)/t6-,7+,8+,9+/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/TrEMBL
DrugBank
GoogleScholar
AffyNet 
| Purchase
DrugBank
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| 8.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ Montpellier
Curated by ChEMBL
| Assay Description
Inhibition of Plasmodium falciparum N-terminal thrombin cleavable His6-tagged ADA expressed in Escherichia coli BL21 assessed as reduction in formati... |
J Med Chem 62: 8365-8391 (2019)
Article DOI: 10.1021/acs.jmedchem.9b00182
BindingDB Entry DOI: 10.7270/Q2T1571N |
More data for this
Ligand-Target Pair | |
Purine nucleoside phosphorylase
(Homo sapiens (Human)) | BDBM50293088

(7-((2R,3R,4S,5S)-3,4-dihydroxy-5-(hydroxymethyl)py...)Show SMILES OC[C@@H]1N[C@@H]([C@@H](O)[C@H]1O)c1c[nH]c2c1nc[nH]c2=O |r| Show InChI InChI=1S/C11H14N4O4/c16-2-5-9(17)10(18)7(15-5)4-1-12-8-6(4)13-3-14-11(8)19/h1,3,5,7,9-10,12,15-18H,2H2,(H,13,14,19)/t5-,7+,9-,10+/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
MMDB
PC cid
PC sid
UniChem
Similars
| MMDB
Article
PubMed
| 9 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ Montpellier
Curated by ChEMBL
| Assay Description
Inhibition of human PNP assessed as inhibitor constant for enzyme-inhibitor-substrate complex formation |
J Med Chem 62: 8365-8391 (2019)
Article DOI: 10.1021/acs.jmedchem.9b00182
BindingDB Entry DOI: 10.7270/Q2T1571N |
More data for this
Ligand-Target Pair | |
Adenosine deaminase
(Homo sapiens (Human)) | BDBM50519494

(CHEMBL1234234)Show SMILES CSC[C@H]1O[C@H]([C@H](O)[C@@H]1O)n1cnc2[C@H](O)CNC=Nc12 |r,c:19| Show InChI InChI=1S/C12H18N4O4S/c1-21-3-7-9(18)10(19)12(20-7)16-5-15-8-6(17)2-13-4-14-11(8)16/h4-7,9-10,12,17-19H,2-3H2,1H3,(H,13,14)/t6-,7-,9-,10-,12-/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
PDB
UniChem
| Article
PubMed
| >10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ Montpellier
Curated by ChEMBL
| Assay Description
Inhibition of human erythrocytes ADA assessed as reduction in formation of inosine using adenosine as substrate |
J Med Chem 62: 8365-8391 (2019)
Article DOI: 10.1021/acs.jmedchem.9b00182
BindingDB Entry DOI: 10.7270/Q2T1571N |
More data for this
Ligand-Target Pair | |
Adenosine deaminase
(Homo sapiens (Human)) | BDBM50367032

(COFORMYCIN)Show SMILES OC[C@H]1O[C@H]([C@H](O)[C@@H]1O)n1cnc2[C@H](O)CNC=Nc12 |r,c:18| Show InChI InChI=1S/C11H16N4O5/c16-2-6-8(18)9(19)11(20-6)15-4-14-7-5(17)1-12-3-13-10(7)15/h3-6,8-9,11,16-19H,1-2H2,(H,12,13)/t5-,6-,8-,9-,11-/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| KEGG
PC cid
PC sid
PDB
UniChem
Similars
| Article
PubMed
| 14 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ Montpellier
Curated by ChEMBL
| Assay Description
Inhibition of human erythrocytes ADA assessed as reduction in formation of inosine using adenosine as substrate |
J Med Chem 62: 8365-8391 (2019)
Article DOI: 10.1021/acs.jmedchem.9b00182
BindingDB Entry DOI: 10.7270/Q2T1571N |
More data for this
Ligand-Target Pair | |
Purine nucleoside phosphorylase
(Plasmodium falciparum) | BDBM50293088

(7-((2R,3R,4S,5S)-3,4-dihydroxy-5-(hydroxymethyl)py...)Show SMILES OC[C@@H]1N[C@@H]([C@@H](O)[C@H]1O)c1c[nH]c2c1nc[nH]c2=O |r| Show InChI InChI=1S/C11H14N4O4/c16-2-5-9(17)10(18)7(15-5)4-1-12-8-6(4)13-3-14-11(8)19/h1,3,5,7,9-10,12,15-18H,2H2,(H,13,14,19)/t5-,7+,9-,10+/m0/s1 | PDB
MMDB
KEGG
UniProtKB/TrEMBL
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
MMDB
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 18 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ Montpellier
Curated by ChEMBL
| Assay Description
Inhibition of Plasmodium falciparum His-tagged PNP assessed as inhibitor constant for enzyme-inhibitor complex formation |
J Med Chem 62: 8365-8391 (2019)
Article DOI: 10.1021/acs.jmedchem.9b00182
BindingDB Entry DOI: 10.7270/Q2T1571N |
More data for this
Ligand-Target Pair | |
Purine nucleoside phosphorylase
(Plasmodium falciparum) | BDBM50195587

(1,4-DIDEOXY-4-AZA-1-(S)-(9-DEAZAHYPOXANTHIN-9-YL)-...)Show SMILES OC[C@H]1N[C@H]([C@H](O)[C@@H]1O)c1c[nH]c2c1nc[nH]c2=O |r| Show InChI InChI=1S/C11H14N4O4/c16-2-5-9(17)10(18)7(15-5)4-1-12-8-6(4)13-3-14-11(8)19/h1,3,5,7,9-10,12,15-18H,2H2,(H,13,14,19)/t5-,7+,9-,10+/m1/s1 | PDB
MMDB
KEGG
UniProtKB/TrEMBL
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
Article
PubMed
| 29 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ Montpellier
Curated by ChEMBL
| Assay Description
Inhibition of Plasmodium falciparum His-tagged PNP assessed as inhibitor constant for enzyme-inhibitor complex formation |
J Med Chem 62: 8365-8391 (2019)
Article DOI: 10.1021/acs.jmedchem.9b00182
BindingDB Entry DOI: 10.7270/Q2T1571N |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Purine nucleoside phosphorylase
(Homo sapiens (Human)) | BDBM50293086

((1S)-1,4-dideoxy-1,4-imino-1-(9-deazaguanin-9-yl)-...)Show SMILES Nc1nc2c(c[nH]c2c(=O)[nH]1)[C@@H]1N[C@H](CO)[C@@H](O)[C@H]1O |r| Show InChI InChI=1S/C11H15N5O4/c12-11-15-5-3(1-13-7(5)10(20)16-11)6-9(19)8(18)4(2-17)14-6/h1,4,6,8-9,13-14,17-19H,2H2,(H3,12,15,16,20)/t4-,6+,8-,9+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| MMDB
Article
PubMed
| 29 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ Montpellier
Curated by ChEMBL
| Assay Description
Inhibition of human PNP assessed as inhibitor constant for enzyme-inhibitor-substrate complex formation |
J Med Chem 62: 8365-8391 (2019)
Article DOI: 10.1021/acs.jmedchem.9b00182
BindingDB Entry DOI: 10.7270/Q2T1571N |
More data for this
Ligand-Target Pair | |
Purine nucleoside phosphorylase
(Homo sapiens (Human)) | BDBM50293088

(7-((2R,3R,4S,5S)-3,4-dihydroxy-5-(hydroxymethyl)py...)Show SMILES OC[C@@H]1N[C@@H]([C@@H](O)[C@H]1O)c1c[nH]c2c1nc[nH]c2=O |r| Show InChI InChI=1S/C11H14N4O4/c16-2-5-9(17)10(18)7(15-5)4-1-12-8-6(4)13-3-14-11(8)19/h1,3,5,7,9-10,12,15-18H,2H2,(H,13,14,19)/t5-,7+,9-,10+/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
MMDB
PC cid
PC sid
UniChem
Similars
| MMDB
Article
PubMed
| 32 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ Montpellier
Curated by ChEMBL
| Assay Description
Inhibition of human PNP assessed as inhibitor constant for enzyme-inhibitor complex formation |
J Med Chem 62: 8365-8391 (2019)
Article DOI: 10.1021/acs.jmedchem.9b00182
BindingDB Entry DOI: 10.7270/Q2T1571N |
More data for this
Ligand-Target Pair | |
Hypoxanthine-guanine phosphoribosyltransferase
(Homo sapiens (Human)) | BDBM50519500

(CHEMBL1233603)Show SMILES Nc1nc2c(c[nH]c2c(=O)[nH]1)[C@@H]1N[C@H](COP(O)(O)=O)[C@@H](O)[C@H]1O |r| Show InChI InChI=1S/C11H16N5O7P/c12-11-15-5-3(1-13-7(5)10(19)16-11)6-9(18)8(17)4(14-6)2-23-24(20,21)22/h1,4,6,8-9,13-14,17-18H,2H2,(H2,20,21,22)(H3,12,15,16,19)/t4-,6+,8-,9+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
PDB
UniChem
| PDB
Article
PubMed
| 56 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ Montpellier
Curated by ChEMBL
| Assay Description
Inhibition of human HGPRT assessed as inhibitor constant for enzyme-inhibitor complex formation using [5'-14C]IMP as substrate by scintillation count... |
J Med Chem 62: 8365-8391 (2019)
Article DOI: 10.1021/acs.jmedchem.9b00182
BindingDB Entry DOI: 10.7270/Q2T1571N |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Purine nucleoside phosphorylase
(Homo sapiens (Human)) | BDBM50195587

(1,4-DIDEOXY-4-AZA-1-(S)-(9-DEAZAHYPOXANTHIN-9-YL)-...)Show SMILES OC[C@H]1N[C@H]([C@H](O)[C@@H]1O)c1c[nH]c2c1nc[nH]c2=O |r| Show InChI InChI=1S/C11H14N4O4/c16-2-5-9(17)10(18)7(15-5)4-1-12-8-6(4)13-3-14-11(8)19/h1,3,5,7,9-10,12,15-18H,2H2,(H,13,14,19)/t5-,7+,9-,10+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| MMDB
PDB
Article
PubMed
| 72 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ Montpellier
Curated by ChEMBL
| Assay Description
Inhibition of human PNP assessed as inhibitor constant for enzyme-inhibitor-substrate complex formation |
J Med Chem 62: 8365-8391 (2019)
Article DOI: 10.1021/acs.jmedchem.9b00182
BindingDB Entry DOI: 10.7270/Q2T1571N |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Purine nucleoside phosphorylase
(Homo sapiens (Human)) | BDBM50247158

(7-(((3S,4S)-3-hydroxy-4-(hydroxymethyl)pyrrolidin-...)Show SMILES OC[C@@H]1CN(Cc2c[nH]c3c2nc[nH]c3=O)C[C@H]1O |r| Show InChI InChI=1S/C12H16N4O3/c17-5-8-3-16(4-9(8)18)2-7-1-13-11-10(7)14-6-15-12(11)19/h1,6,8-9,13,17-18H,2-5H2,(H,14,15,19)/t8-,9+/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
MMDB
PC cid
PC sid
UniChem
Similars
| MMDB
Article
PubMed
| 80 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ Montpellier
Curated by ChEMBL
| Assay Description
Inhibition of human PNP assessed as inhibitor constant for enzyme-inhibitor-substrate complex formation |
J Med Chem 62: 8365-8391 (2019)
Article DOI: 10.1021/acs.jmedchem.9b00182
BindingDB Entry DOI: 10.7270/Q2T1571N |
More data for this
Ligand-Target Pair | |
Hypoxanthine-guanine phosphoribosyltransferase
(Homo sapiens (Human)) | BDBM50519497

(CHEMBL4546337)Show SMILES Nc1nc2n(C[C@H](CO)n3cc(nn3)P(O)(O)=O)cnc2c(=O)[nH]1 |r| Show InChI InChI=1S/C10H13N8O5P/c11-10-13-8-7(9(20)14-10)12-4-17(8)1-5(3-19)18-2-6(15-16-18)24(21,22)23/h2,4-5,19H,1,3H2,(H2,21,22,23)(H3,11,13,14,20)/t5-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ Montpellier
Curated by ChEMBL
| Assay Description
Inhibition of human HGPRT using Prib-PP as substrate by Hanes-plot based method |
J Med Chem 62: 8365-8391 (2019)
Article DOI: 10.1021/acs.jmedchem.9b00182
BindingDB Entry DOI: 10.7270/Q2T1571N |
More data for this
Ligand-Target Pair | |
Hypoxanthine-guanine phosphoribosyltransferase
(Homo sapiens (Human)) | BDBM50519492

(CHEMBL1233663)Show SMILES O[C@@H]1[C@@H](COP(O)(O)=O)N[C@H]([C@@H]1O)c1c[nH]c2c1nc[nH]c2=O |r| Show InChI InChI=1S/C11H15N4O7P/c16-9-5(2-22-23(19,20)21)15-7(10(9)17)4-1-12-8-6(4)13-3-14-11(8)18/h1,3,5,7,9-10,12,15-17H,2H2,(H,13,14,18)(H2,19,20,21)/t5-,7+,9-,10+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
PDB
UniChem
| Article
PubMed
| 250 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ Montpellier
Curated by ChEMBL
| Assay Description
Inhibition of human HGPRT assessed as inhibitor constant for enzyme-inhibitor complex formation using [5'-14C]IMP as substrate by scintillation count... |
J Med Chem 62: 8365-8391 (2019)
Article DOI: 10.1021/acs.jmedchem.9b00182
BindingDB Entry DOI: 10.7270/Q2T1571N |
More data for this
Ligand-Target Pair | |
Purine nucleoside phosphorylase
(Homo sapiens (Human)) | BDBM50247149

(5'-Methylthio-ImmH | CHEMBL473929 | US9290501, (A))Show SMILES CSC[C@H]1N[C@H]([C@H](O)[C@@H]1O)c1c[nH]c2c1nc[nH]c2=O |r| Show InChI InChI=1S/C12H16N4O3S/c1-20-3-6-10(17)11(18)8(16-6)5-2-13-9-7(5)14-4-15-12(9)19/h2,4,6,8,10-11,13,16-18H,3H2,1H3,(H,14,15,19)/t6-,8+,10-,11+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
MMDB
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 303 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ Montpellier
Curated by ChEMBL
| Assay Description
Inhibition of human PNP expressed in Escherichia coli assessed as reduction in uric acid formation by spectrophotometric method |
J Med Chem 62: 8365-8391 (2019)
Article DOI: 10.1021/acs.jmedchem.9b00182
BindingDB Entry DOI: 10.7270/Q2T1571N |
More data for this
Ligand-Target Pair | |
Hypoxanthine-guanine phosphoribosyltransferase
(Homo sapiens (Human)) | BDBM50519504
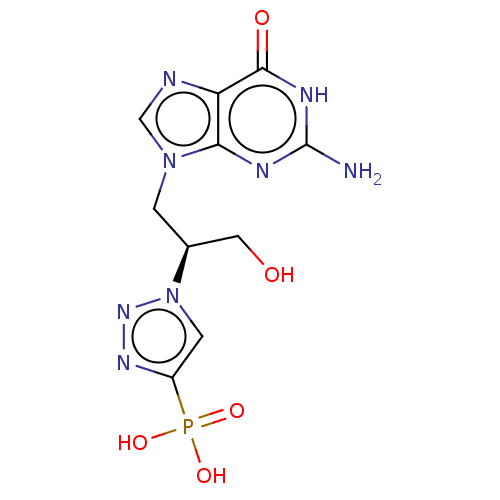
(CHEMBL4561412)Show SMILES Nc1nc2n(C[C@@H](CO)n3cc(nn3)P(O)(O)=O)cnc2c(=O)[nH]1 |r| Show InChI InChI=1S/C10H13N8O5P/c11-10-13-8-7(9(20)14-10)12-4-17(8)1-5(3-19)18-2-6(15-16-18)24(21,22)23/h2,4-5,19H,1,3H2,(H2,21,22,23)(H3,11,13,14,20)/t5-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 400 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ Montpellier
Curated by ChEMBL
| Assay Description
Inhibition of human HGPRT using Prib-PP as substrate by Hanes-plot based method |
J Med Chem 62: 8365-8391 (2019)
Article DOI: 10.1021/acs.jmedchem.9b00182
BindingDB Entry DOI: 10.7270/Q2T1571N |
More data for this
Ligand-Target Pair | |
Hypoxanthine-guanine phosphoribosyltransferase
(Homo sapiens (Human)) | BDBM50519501
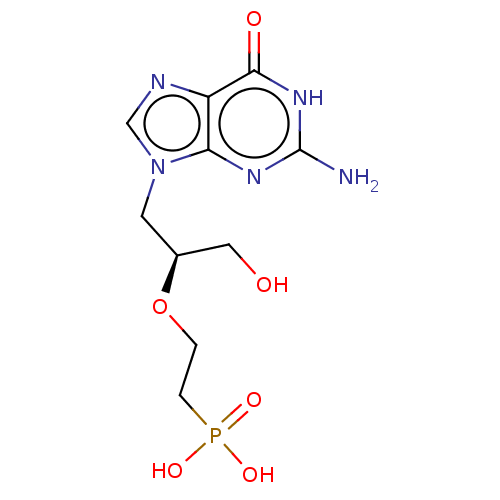
(CHEMBL4583093)Show SMILES Nc1nc2n(C[C@@H](CO)OCCP(O)(O)=O)cnc2c(=O)[nH]1 |r| Show InChI InChI=1S/C10H16N5O6P/c11-10-13-8-7(9(17)14-10)12-5-15(8)3-6(4-16)21-1-2-22(18,19)20/h5-6,16H,1-4H2,(H2,18,19,20)(H3,11,13,14,17)/t6-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
PDB
UniChem
| PDB
Article
PubMed
| 600 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ Montpellier
Curated by ChEMBL
| Assay Description
Inhibition of human N-terminal His6-tagged HGPRT using Prib-PP as substrate by Hanes-plot based method |
J Med Chem 62: 8365-8391 (2019)
Article DOI: 10.1021/acs.jmedchem.9b00182
BindingDB Entry DOI: 10.7270/Q2T1571N |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Purine nucleoside phosphorylase
(Homo sapiens (Human)) | BDBM50247158

(7-(((3S,4S)-3-hydroxy-4-(hydroxymethyl)pyrrolidin-...)Show SMILES OC[C@@H]1CN(Cc2c[nH]c3c2nc[nH]c3=O)C[C@H]1O |r| Show InChI InChI=1S/C12H16N4O3/c17-5-8-3-16(4-9(8)18)2-7-1-13-11-10(7)14-6-15-12(11)19/h1,6,8-9,13,17-18H,2-5H2,(H,14,15,19)/t8-,9+/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
MMDB
PC cid
PC sid
UniChem
Similars
| MMDB
Article
PubMed
| 1.70E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ Montpellier
Curated by ChEMBL
| Assay Description
Inhibition of human PNP assessed as inhibitor constant for enzyme-inhibitor complex formation |
J Med Chem 62: 8365-8391 (2019)
Article DOI: 10.1021/acs.jmedchem.9b00182
BindingDB Entry DOI: 10.7270/Q2T1571N |
More data for this
Ligand-Target Pair | |
Hypoxanthine-guanine phosphoribosyltransferase
(Homo sapiens (Human)) | BDBM50519505
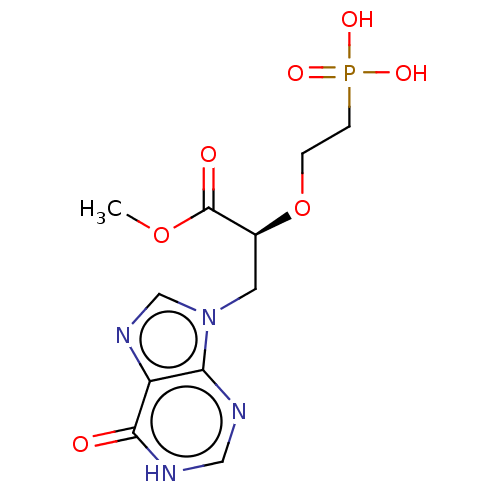
(CHEMBL4578350)Show SMILES COC(=O)[C@H](Cn1cnc2c1nc[nH]c2=O)OCCP(O)(O)=O |r| Show InChI InChI=1S/C11H15N4O7P/c1-21-11(17)7(22-2-3-23(18,19)20)4-15-6-14-8-9(15)12-5-13-10(8)16/h5-7H,2-4H2,1H3,(H,12,13,16)(H2,18,19,20)/t7-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 6.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ Montpellier
Curated by ChEMBL
| Assay Description
Inhibition of human N-terminal His6-tagged HGPRT using Prib-PP as substrate by Hanes-plot based method |
J Med Chem 62: 8365-8391 (2019)
Article DOI: 10.1021/acs.jmedchem.9b00182
BindingDB Entry DOI: 10.7270/Q2T1571N |
More data for this
Ligand-Target Pair | |
Hypoxanthine-guanine phosphoribosyltransferase
(Homo sapiens (Human)) | BDBM50519493
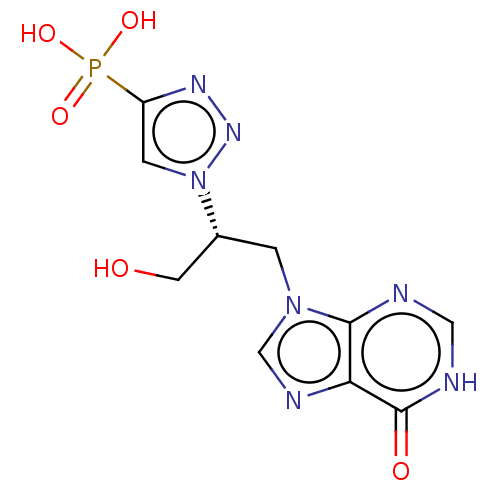
(CHEMBL4453040)Show SMILES OC[C@@H](Cn1cnc2c1nc[nH]c2=O)n1cc(nn1)P(O)(O)=O |r| Show InChI InChI=1S/C10H12N7O5P/c18-3-6(17-2-7(14-15-17)23(20,21)22)1-16-5-13-8-9(16)11-4-12-10(8)19/h2,4-6,18H,1,3H2,(H,11,12,19)(H2,20,21,22)/t6-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 8.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ Montpellier
Curated by ChEMBL
| Assay Description
Inhibition of human HGPRT using Prib-PP as substrate by Hanes-plot based method |
J Med Chem 62: 8365-8391 (2019)
Article DOI: 10.1021/acs.jmedchem.9b00182
BindingDB Entry DOI: 10.7270/Q2T1571N |
More data for this
Ligand-Target Pair | |
Hypoxanthine-guanine phosphoribosyltransferase
(Homo sapiens (Human)) | BDBM50519502
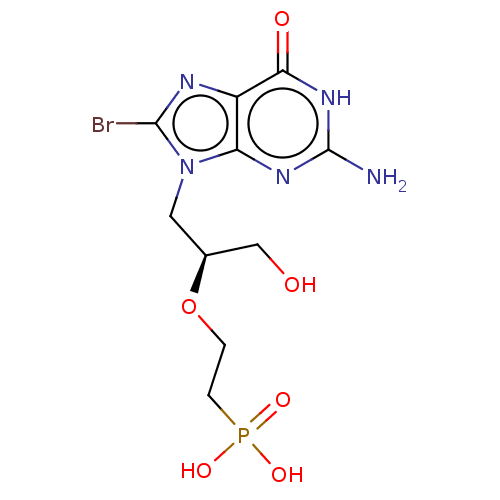
(CHEMBL4452934)Show SMILES Nc1nc2n(C[C@@H](CO)OCCP(O)(O)=O)c(Br)nc2c(=O)[nH]1 |r| Show InChI InChI=1S/C10H15BrN5O6P/c11-9-13-6-7(14-10(12)15-8(6)18)16(9)3-5(4-17)22-1-2-23(19,20)21/h5,17H,1-4H2,(H2,19,20,21)(H3,12,14,15,18)/t5-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 1.30E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ Montpellier
Curated by ChEMBL
| Assay Description
Inhibition of human N-terminal His6-tagged HGPRT using Prib-PP as substrate by Hanes-plot based method |
J Med Chem 62: 8365-8391 (2019)
Article DOI: 10.1021/acs.jmedchem.9b00182
BindingDB Entry DOI: 10.7270/Q2T1571N |
More data for this
Ligand-Target Pair | |
Hypoxanthine-guanine phosphoribosyltransferase
(Homo sapiens (Human)) | BDBM50519496
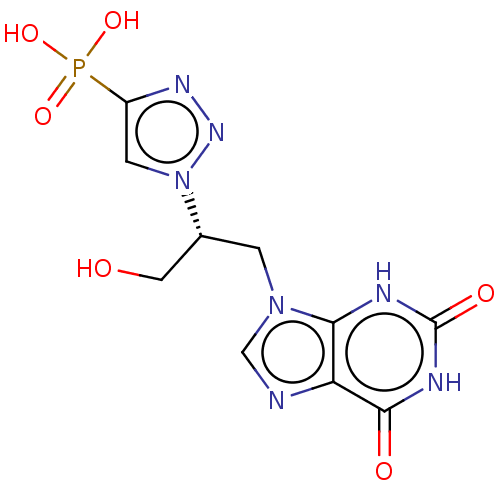
(CHEMBL4439813)Show SMILES OC[C@@H](Cn1cnc2c1[nH]c(=O)[nH]c2=O)n1cc(nn1)P(O)(O)=O |r| Show InChI InChI=1S/C10H12N7O6P/c18-3-5(17-2-6(14-15-17)24(21,22)23)1-16-4-11-7-8(16)12-10(20)13-9(7)19/h2,4-5,18H,1,3H2,(H2,21,22,23)(H2,12,13,19,20)/t5-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| >5.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ Montpellier
Curated by ChEMBL
| Assay Description
Inhibition of human HGPRT using Prib-PP as substrate by Hanes-plot based method |
J Med Chem 62: 8365-8391 (2019)
Article DOI: 10.1021/acs.jmedchem.9b00182
BindingDB Entry DOI: 10.7270/Q2T1571N |
More data for this
Ligand-Target Pair | |
Hypoxanthine-guanine phosphoribosyltransferase
(Homo sapiens (Human)) | BDBM50519503
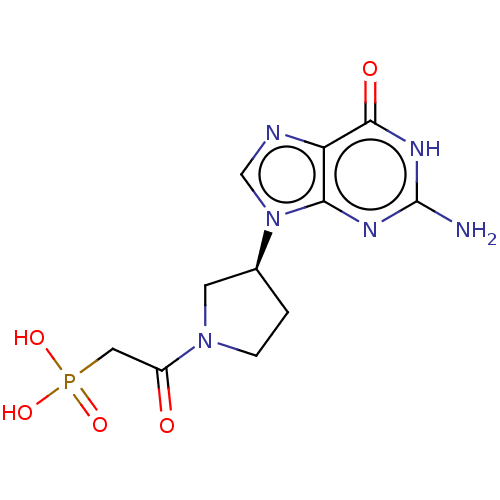
(CHEMBL2420976)Show SMILES Nc1nc2n(cnc2c(=O)[nH]1)[C@H]1CCN(C1)C(=O)CP(O)(O)=O |r| Show InChI InChI=1S/C11H15N6O5P/c12-11-14-9-8(10(19)15-11)13-5-17(9)6-1-2-16(3-6)7(18)4-23(20,21)22/h5-6H,1-4H2,(H2,20,21,22)(H3,12,14,15,19)/t6-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
PDB
UniChem
| Article
PubMed
| 7.30E+7 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ Montpellier
Curated by ChEMBL
| Assay Description
Inhibition of human HGPRT using Prib-PP as substrate by Hanes-plot based method |
J Med Chem 62: 8365-8391 (2019)
Article DOI: 10.1021/acs.jmedchem.9b00182
BindingDB Entry DOI: 10.7270/Q2T1571N |
More data for this
Ligand-Target Pair | |
GMP synthase [glutamine-hydrolyzing]
(Homo sapiens) | BDBM50519499
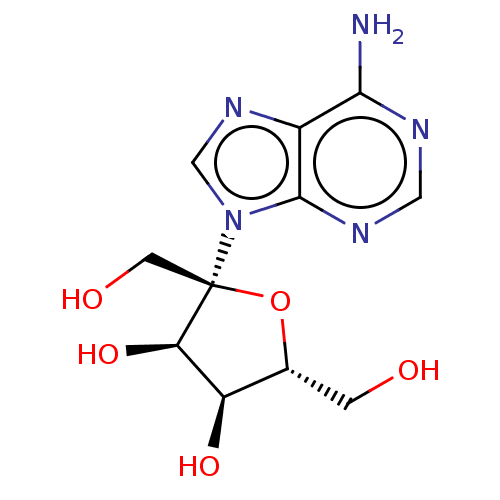
(CHEBI:8612 | Psicofuranine)Show SMILES Nc1ncnc2n(cnc12)[C@]1(CO)O[C@H](CO)[C@@H](O)[C@H]1O |r| Show InChI InChI=1S/C11H15N5O5/c12-9-6-10(14-3-13-9)16(4-15-6)11(2-18)8(20)7(19)5(1-17)21-11/h3-5,7-8,17-20H,1-2H2,(H2,12,13,14)/t5-,7-,8-,11-/m1/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
KEGG
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1.73E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ Montpellier
Curated by ChEMBL
| Assay Description
Inhibition of human GMP synthase |
J Med Chem 62: 8365-8391 (2019)
Article DOI: 10.1021/acs.jmedchem.9b00182
BindingDB Entry DOI: 10.7270/Q2T1571N |
More data for this
Ligand-Target Pair | |
GMP synthase [glutamine-hydrolyzing]
(Homo sapiens) | BDBM50519498
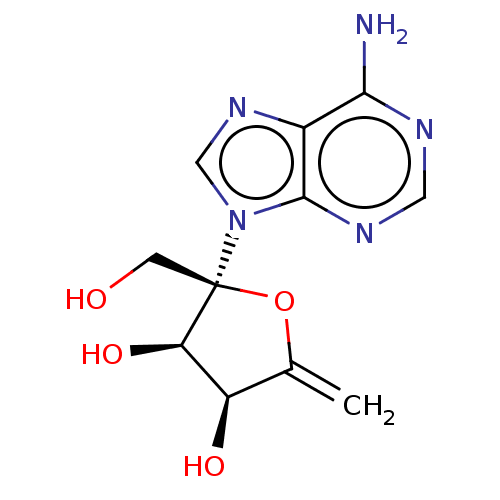
(Decoyinine)Show SMILES Nc1ncnc2n(cnc12)[C@]1(CO)OC(=C)[C@@H](O)[C@H]1O Show InChI InChI=1S/C11H13N5O4/c1-5-7(18)8(19)11(2-17,20-5)16-4-15-6-9(12)13-3-14-10(6)16/h3-4,7-8,17-19H,1-2H2,(H2,12,13,14)/t7-,8-,11-/m1/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 4.65E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ Montpellier
Curated by ChEMBL
| Assay Description
Inhibition of human GMP synthase |
J Med Chem 62: 8365-8391 (2019)
Article DOI: 10.1021/acs.jmedchem.9b00182
BindingDB Entry DOI: 10.7270/Q2T1571N |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data