

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Eukaryotic initiation factor 4A-I (Homo sapiens (Human)) | BDBM50540861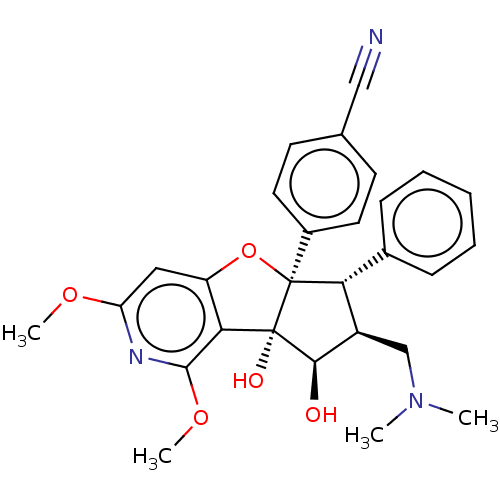 (EFT-226 | Eft226 | Zotatifin) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem | Article PubMed | n/a | n/a | 1.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Inception Therapeutics Curated by ChEMBL | Assay Description Inhibition of eIF4A1 in human MDA-MB-231 cells assessed as inhibition of cellular-translation incubated for 4 hrs by specific tandem sequence motif r... | J Med Chem 63: 5879-5955 (2020) Article DOI: 10.1021/acs.jmedchem.0c00182 BindingDB Entry DOI: 10.7270/Q2MC93K8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Eukaryotic initiation factor 4A-I (Homo sapiens (Human)) | BDBM50196926 (CHEMBL438139 | Rocaglamide | US10085988, Compound ...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | n/a | n/a | 4.70 | n/a | n/a | n/a | n/a | n/a | n/a |
Inception Therapeutics Curated by ChEMBL | Assay Description Inhibition of eIF4A1 in human MDA-MB-231 cells assessed as inhibition of cellular-translation incubated for 4 hrs by specific tandem sequence motif r... | J Med Chem 63: 5879-5955 (2020) Article DOI: 10.1021/acs.jmedchem.0c00182 BindingDB Entry DOI: 10.7270/Q2MC93K8 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Eukaryotic initiation factor 4A-I (Homo sapiens (Human)) | BDBM50540861 (EFT-226 | Eft226 | Zotatifin) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem | Article PubMed | n/a | n/a | 6.30 | n/a | n/a | n/a | n/a | n/a | n/a |
Inception Therapeutics Curated by ChEMBL | Assay Description Inhibition of eIF4A1 in human HAP1 cells assessed as reduction in cell proliferation incubated for 72 hrs by CellTiter-Glo reagent based assay | J Med Chem 63: 5879-5955 (2020) Article DOI: 10.1021/acs.jmedchem.0c00182 BindingDB Entry DOI: 10.7270/Q2MC93K8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Eukaryotic initiation factor 4A-I (Homo sapiens (Human)) | BDBM50540861 (EFT-226 | Eft226 | Zotatifin) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem | Article PubMed | n/a | n/a | 14 | n/a | n/a | n/a | n/a | n/a | n/a |
Inception Therapeutics Curated by ChEMBL | Assay Description Inhibition of eIF4A1 in human MDA-MB-231 cells assessed as inhibition of cellular-translation incubated for 4 hrs by specific tandem sequence motif r... | J Med Chem 63: 5879-5955 (2020) Article DOI: 10.1021/acs.jmedchem.0c00182 BindingDB Entry DOI: 10.7270/Q2MC93K8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Eukaryotic initiation factor 4A-I (Homo sapiens (Human)) | BDBM50196926 (CHEMBL438139 | Rocaglamide | US10085988, Compound ...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | n/a | n/a | 47 | n/a | n/a | n/a | n/a | n/a | n/a |
Inception Therapeutics Curated by ChEMBL | Assay Description Inhibition of eIF4A1 in human MDA-MB-231 cells assessed as inhibition of cellular-translation incubated for 4 hrs by specific tandem sequence motif r... | J Med Chem 63: 5879-5955 (2020) Article DOI: 10.1021/acs.jmedchem.0c00182 BindingDB Entry DOI: 10.7270/Q2MC93K8 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Eukaryotic initiation factor 4A-I (Homo sapiens (Human)) | BDBM50080528 (3-((R)-2-((1S,3S,5S)-3,5-dimethyl-2-oxocyclohexyl)...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | Purchase CHEMBL KEGG PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | 87 | n/a | n/a | n/a | n/a | n/a | n/a |
Inception Therapeutics Curated by ChEMBL | Assay Description Inhibition of eIF4A1 in human MDA-MB-231 cells assessed as inhibition of cellular-translation incubated for 4 hrs by specific tandem sequence motif r... | J Med Chem 63: 5879-5955 (2020) Article DOI: 10.1021/acs.jmedchem.0c00182 BindingDB Entry DOI: 10.7270/Q2MC93K8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Eukaryotic initiation factor 4A-I (Homo sapiens (Human)) | BDBM50540861 (EFT-226 | Eft226 | Zotatifin) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem | Article PubMed | n/a | n/a | 92 | n/a | n/a | n/a | n/a | n/a | n/a |
Inception Therapeutics Curated by ChEMBL | Assay Description Inhibition of eIF4A1 in human MDA-MB-231 cells assessed as inhibition of cellular-translation incubated for 4 hrs by specific tandem sequence motif r... | J Med Chem 63: 5879-5955 (2020) Article DOI: 10.1021/acs.jmedchem.0c00182 BindingDB Entry DOI: 10.7270/Q2MC93K8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Eukaryotic initiation factor 4A-I (Homo sapiens (Human)) | BDBM50080528 (3-((R)-2-((1S,3S,5S)-3,5-dimethyl-2-oxocyclohexyl)...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | Purchase CHEMBL KEGG PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | 101 | n/a | n/a | n/a | n/a | n/a | n/a |
Inception Therapeutics Curated by ChEMBL | Assay Description Inhibition of eIF4A1 in human MDA-MB-231 cells assessed as inhibition of cellular-translation incubated for 4 hrs by specific tandem sequence motif r... | J Med Chem 63: 5879-5955 (2020) Article DOI: 10.1021/acs.jmedchem.0c00182 BindingDB Entry DOI: 10.7270/Q2MC93K8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Eukaryotic initiation factor 4A-I (Homo sapiens (Human)) | BDBM50196926 (CHEMBL438139 | Rocaglamide | US10085988, Compound ...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | n/a | n/a | 135 | n/a | n/a | n/a | n/a | n/a | n/a |
Inception Therapeutics Curated by ChEMBL | Assay Description Inhibition of eIF4A1 in human MDA-MB-231 cells assessed as inhibition of cellular-translation incubated for 4 hrs by specific tandem sequence motif r... | J Med Chem 63: 5879-5955 (2020) Article DOI: 10.1021/acs.jmedchem.0c00182 BindingDB Entry DOI: 10.7270/Q2MC93K8 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Eukaryotic initiation factor 4A-I (Homo sapiens (Human)) | BDBM50080528 (3-((R)-2-((1S,3S,5S)-3,5-dimethyl-2-oxocyclohexyl)...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | Purchase CHEMBL KEGG PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | 142 | n/a | n/a | n/a | n/a | n/a | n/a |
Inception Therapeutics Curated by ChEMBL | Assay Description Inhibition of eIF4A1 in human MDA-MB-231 cells assessed as inhibition of cellular-translation incubated for 4 hrs by specific tandem sequence motif r... | J Med Chem 63: 5879-5955 (2020) Article DOI: 10.1021/acs.jmedchem.0c00182 BindingDB Entry DOI: 10.7270/Q2MC93K8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Eukaryotic initiation factor 4A-I (Homo sapiens (Human)) | BDBM50235738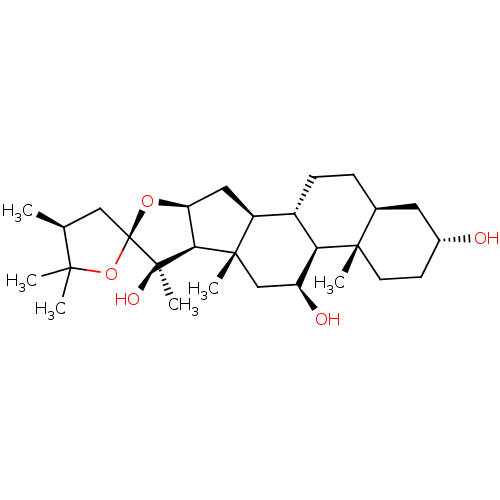 (Hippuristanol) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 204 | n/a | n/a | n/a | n/a | n/a | n/a |
Inception Therapeutics Curated by ChEMBL | Assay Description Inhibition of eIF4A1 in human MDA-MB-231 cells assessed as inhibition of cellular-translation incubated for 4 hrs by specific tandem sequence motif r... | J Med Chem 63: 5879-5955 (2020) Article DOI: 10.1021/acs.jmedchem.0c00182 BindingDB Entry DOI: 10.7270/Q2MC93K8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Eukaryotic initiation factor 4A-I (Homo sapiens (Human)) | BDBM50235738 (Hippuristanol) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 210 | n/a | n/a | n/a | n/a | n/a | n/a |
Inception Therapeutics Curated by ChEMBL | Assay Description Inhibition of eIF4A1 in human MDA-MB-231 cells assessed as inhibition of cellular-translation incubated for 4 hrs by specific tandem sequence motif r... | J Med Chem 63: 5879-5955 (2020) Article DOI: 10.1021/acs.jmedchem.0c00182 BindingDB Entry DOI: 10.7270/Q2MC93K8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Eukaryotic initiation factor 4A-I (Homo sapiens (Human)) | BDBM50540861 (EFT-226 | Eft226 | Zotatifin) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem | Article PubMed | n/a | n/a | 218 | n/a | n/a | n/a | n/a | n/a | n/a |
Inception Therapeutics Curated by ChEMBL | Assay Description Inhibition of eIF4A1 in human MDA-MB-231 cells assessed as inhibition of cellular-translation incubated for 4 hrs by specific tandem sequence motif r... | J Med Chem 63: 5879-5955 (2020) Article DOI: 10.1021/acs.jmedchem.0c00182 BindingDB Entry DOI: 10.7270/Q2MC93K8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Eukaryotic initiation factor 4A-I (Homo sapiens (Human)) | BDBM50080528 (3-((R)-2-((1S,3S,5S)-3,5-dimethyl-2-oxocyclohexyl)...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | Purchase CHEMBL KEGG PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | 226 | n/a | n/a | n/a | n/a | n/a | n/a |
Inception Therapeutics Curated by ChEMBL | Assay Description Inhibition of eIF4A1 in human MDA-MB-231 cells assessed as inhibition of cellular-translation incubated for 4 hrs by specific tandem sequence motif r... | J Med Chem 63: 5879-5955 (2020) Article DOI: 10.1021/acs.jmedchem.0c00182 BindingDB Entry DOI: 10.7270/Q2MC93K8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Eukaryotic initiation factor 4A-I (Homo sapiens (Human)) | BDBM50196926 (CHEMBL438139 | Rocaglamide | US10085988, Compound ...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | n/a | n/a | 231 | n/a | n/a | n/a | n/a | n/a | n/a |
Inception Therapeutics Curated by ChEMBL | Assay Description Inhibition of eIF4A1 in human MDA-MB-231 cells assessed as inhibition of cellular-translation incubated for 4 hrs by specific tandem sequence motif r... | J Med Chem 63: 5879-5955 (2020) Article DOI: 10.1021/acs.jmedchem.0c00182 BindingDB Entry DOI: 10.7270/Q2MC93K8 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Eukaryotic initiation factor 4A-I (Homo sapiens (Human)) | BDBM50235738 (Hippuristanol) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 369 | n/a | n/a | n/a | n/a | n/a | n/a |
Inception Therapeutics Curated by ChEMBL | Assay Description Inhibition of eIF4A1 in human MDA-MB-231 cells assessed as inhibition of cellular-translation incubated for 4 hrs by specific tandem sequence motif r... | J Med Chem 63: 5879-5955 (2020) Article DOI: 10.1021/acs.jmedchem.0c00182 BindingDB Entry DOI: 10.7270/Q2MC93K8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Eukaryotic initiation factor 4A-I (Homo sapiens (Human)) | BDBM50235738 (Hippuristanol) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 426 | n/a | n/a | n/a | n/a | n/a | n/a |
Inception Therapeutics Curated by ChEMBL | Assay Description Inhibition of eIF4A1 in human MDA-MB-231 cells assessed as inhibition of cellular-translation incubated for 4 hrs by specific tandem sequence motif r... | J Med Chem 63: 5879-5955 (2020) Article DOI: 10.1021/acs.jmedchem.0c00182 BindingDB Entry DOI: 10.7270/Q2MC93K8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Potassium voltage-gated channel subfamily H member 2 (Homo sapiens (Human)) | BDBM50540859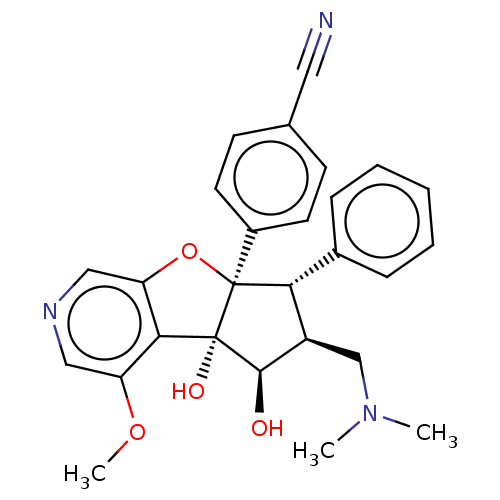 (CHEMBL4632904) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Inception Therapeutics Curated by ChEMBL | Assay Description Inhibition of human ERG by patch-clamp assay | J Med Chem 63: 5879-5955 (2020) Article DOI: 10.1021/acs.jmedchem.0c00182 BindingDB Entry DOI: 10.7270/Q2MC93K8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Potassium voltage-gated channel subfamily H member 2 (Homo sapiens (Human)) | BDBM50540860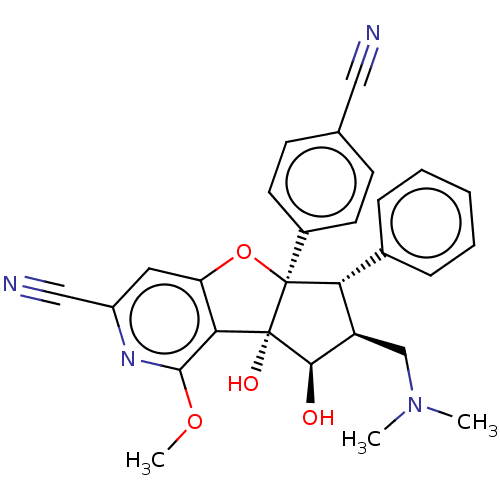 (CHEMBL4645423) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Inception Therapeutics Curated by ChEMBL | Assay Description Inhibition of human ERG by patch-clamp assay | J Med Chem 63: 5879-5955 (2020) Article DOI: 10.1021/acs.jmedchem.0c00182 BindingDB Entry DOI: 10.7270/Q2MC93K8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Potassium voltage-gated channel subfamily H member 2 (Homo sapiens (Human)) | BDBM50540861 (EFT-226 | Eft226 | Zotatifin) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem | Article PubMed | n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Inception Therapeutics Curated by ChEMBL | Assay Description Inhibition of human ERG by patch-clamp assay | J Med Chem 63: 5879-5955 (2020) Article DOI: 10.1021/acs.jmedchem.0c00182 BindingDB Entry DOI: 10.7270/Q2MC93K8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Eukaryotic initiation factor 4A-I (Homo sapiens (Human)) | BDBM50540861 (EFT-226 | Eft226 | Zotatifin) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem | Article PubMed | n/a | n/a | n/a | 3.19E+3 | n/a | n/a | n/a | n/a | n/a |
Inception Therapeutics Curated by ChEMBL | Assay Description Binding affinity to full length recombinant eIF4A1 (unknown origin) assessed as induction ternary complex formation in presence of AGAGAG by measurin... | J Med Chem 63: 5879-5955 (2020) Article DOI: 10.1021/acs.jmedchem.0c00182 BindingDB Entry DOI: 10.7270/Q2MC93K8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Eukaryotic initiation factor 4A-I (Homo sapiens (Human)) | BDBM50540861 (EFT-226 | Eft226 | Zotatifin) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem | Article PubMed | n/a | n/a | n/a | 9.60E+3 | n/a | n/a | n/a | n/a | n/a |
Inception Therapeutics Curated by ChEMBL | Assay Description Binding affinity to full length recombinant eIF4A1 (unknown origin) assessed as induction ternary complex formation in presence of AGAGAG by measurin... | J Med Chem 63: 5879-5955 (2020) Article DOI: 10.1021/acs.jmedchem.0c00182 BindingDB Entry DOI: 10.7270/Q2MC93K8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Eukaryotic initiation factor 4A-I (Homo sapiens (Human)) | BDBM50196926 (CHEMBL438139 | Rocaglamide | US10085988, Compound ...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | n/a | n/a | n/a | 156 | n/a | n/a | n/a | n/a | n/a |
Inception Therapeutics Curated by ChEMBL | Assay Description Binding affinity to full length recombinant eIF4A1 (unknown origin) assessed as induction ternary complex formation in presence of AGAGAG by measurin... | J Med Chem 63: 5879-5955 (2020) Article DOI: 10.1021/acs.jmedchem.0c00182 BindingDB Entry DOI: 10.7270/Q2MC93K8 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Eukaryotic initiation factor 4A-I (Homo sapiens (Human)) | BDBM50540861 (EFT-226 | Eft226 | Zotatifin) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem | Article PubMed | n/a | n/a | n/a | 21 | n/a | n/a | n/a | n/a | n/a |
Inception Therapeutics Curated by ChEMBL | Assay Description Binding affinity to full length recombinant eIF4A1 (unknown origin) assessed as induction ternary complex formation in presence of AGAGAG by measurin... | J Med Chem 63: 5879-5955 (2020) Article DOI: 10.1021/acs.jmedchem.0c00182 BindingDB Entry DOI: 10.7270/Q2MC93K8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Eukaryotic initiation factor 4A-I (Homo sapiens (Human)) | BDBM50540861 (EFT-226 | Eft226 | Zotatifin) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem | Article PubMed | n/a | n/a | n/a | 2.43E+3 | n/a | n/a | n/a | n/a | n/a |
Inception Therapeutics Curated by ChEMBL | Assay Description Binding affinity to full length recombinant eIF4A1 (unknown origin) assessed as induction ternary complex formation in presence of AGAGAG by measurin... | J Med Chem 63: 5879-5955 (2020) Article DOI: 10.1021/acs.jmedchem.0c00182 BindingDB Entry DOI: 10.7270/Q2MC93K8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||