 Found 20 hits Enz. Inhib. hit(s) with all data for entry = 50011291
Found 20 hits Enz. Inhib. hit(s) with all data for entry = 50011291 Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Leucine-rich repeat serine/threonine-protein kinase 2
(Homo sapiens (Human)) | BDBM50448118
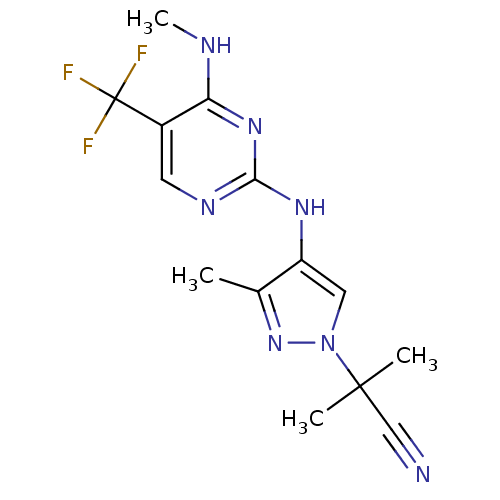
(CHEMBL3122113 | US10590114, No. 80 | US11111235, N...)Show InChI InChI=1S/C14H16F3N7/c1-8-10(6-24(23-8)13(2,3)7-18)21-12-20-5-9(14(15,16)17)11(19-4)22-12/h5-6H,1-4H3,(H2,19,20,21,22) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
PDB
UniChem
Similars
| PDB
Article
PubMed
| 0.600 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of LRRK2 G2019S mutant (unknown origin) |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.0c00093
BindingDB Entry DOI: 10.7270/Q2MC93N5 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Leucine-rich repeat serine/threonine-protein kinase 2
(Homo sapiens (Human)) | BDBM50398668
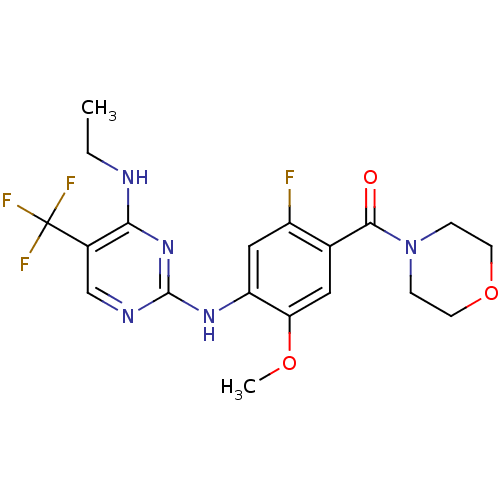
(CHEMBL2178134 | US8802674, 256)Show SMILES CCNc1nc(Nc2cc(F)c(cc2OC)C(=O)N2CCOCC2)ncc1C(F)(F)F Show InChI InChI=1S/C19H21F4N5O3/c1-3-24-16-12(19(21,22)23)10-25-18(27-16)26-14-9-13(20)11(8-15(14)30-2)17(29)28-4-6-31-7-5-28/h8-10H,3-7H2,1-2H3,(H2,24,25,26,27) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
PDB
UniChem
Similars
| Article
PubMed
| 1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of LRRK2 G2019S mutant (unknown origin) |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.0c00093
BindingDB Entry DOI: 10.7270/Q2MC93N5 |
More data for this
Ligand-Target Pair | |
Androgen receptor
(Homo sapiens (Human)) | BDBM50432188

(CHEMBL2346976)Show SMILES CC(=O)N1CCN(CCOc2ccc(cc2)C2CCN(CC2)C2=Nn3c(CC2)nnc3C(F)(F)F)CC1 |t:24| Show InChI InChI=1S/C25H32F3N7O2/c1-18(36)33-14-12-32(13-15-33)16-17-37-21-4-2-19(3-5-21)20-8-10-34(11-9-20)23-7-6-22-29-30-24(25(26,27)28)35(22)31-23/h2-5,20H,6-17H2,1H3 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
| Article
PubMed
| 2.20E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of full length androgen receptor (unknown origin) |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.0c00093
BindingDB Entry DOI: 10.7270/Q2MC93N5 |
More data for this
Ligand-Target Pair | |
Androgen receptor
(Homo sapiens (Human)) | BDBM50432188

(CHEMBL2346976)Show SMILES CC(=O)N1CCN(CCOc2ccc(cc2)C2CCN(CC2)C2=Nn3c(CC2)nnc3C(F)(F)F)CC1 |t:24| Show InChI InChI=1S/C25H32F3N7O2/c1-18(36)33-14-12-32(13-15-33)16-17-37-21-4-2-19(3-5-21)20-8-10-34(11-9-20)23-7-6-22-29-30-24(25(26,27)28)35(22)31-23/h2-5,20H,6-17H2,1H3 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
| Article
PubMed
| 5.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of androgen receptor LBD domain (unknown origin) |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.0c00093
BindingDB Entry DOI: 10.7270/Q2MC93N5 |
More data for this
Ligand-Target Pair | |
Leucine-rich repeat serine/threonine-protein kinase 2
(Homo sapiens (Human)) | BDBM257207
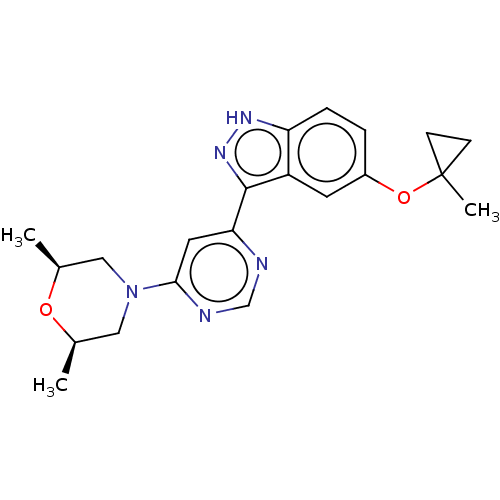
(US9493440, 51)Show SMILES C[C@H]1CN(C[C@@H](C)O1)c1cc(ncn1)-c1n[nH]c2ccc(OC3(C)CC3)cc12 |r| | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
PDB
UniChem
| PDB
Article
PubMed
| n/a | n/a | 0.800 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of recombinant human GST-tagged LRRK2 G2019S mutant catalytic domain (970 to 2527 residues) expressed in baculovirus expression system usi... |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.0c00093
BindingDB Entry DOI: 10.7270/Q2MC93N5 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Aurora kinase A
(Homo sapiens (Human)) | BDBM50277545

(4-(9-chloro-7-(2-fluoro-6-methoxyphenyl)-5H-benzo[...)Show SMILES COc1cc(Nc2ncc3CN=C(c4cc(Cl)ccc4-c3n2)c2c(F)cccc2OC)ccc1C(O)=O |c:11| Show InChI InChI=1S/C27H20ClFN4O4/c1-36-21-5-3-4-20(29)23(21)25-19-10-15(28)6-8-17(19)24-14(12-30-25)13-31-27(33-24)32-16-7-9-18(26(34)35)22(11-16)37-2/h3-11,13H,12H2,1-2H3,(H,34,35)(H,31,32,33) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 1.20 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of Aurora A (unknown origin) |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.0c00093
BindingDB Entry DOI: 10.7270/Q2MC93N5 |
More data for this
Ligand-Target Pair | 
3D Structure (docked) |
Tyrosine-protein kinase JAK3
(Homo sapiens (Human)) | BDBM50234898
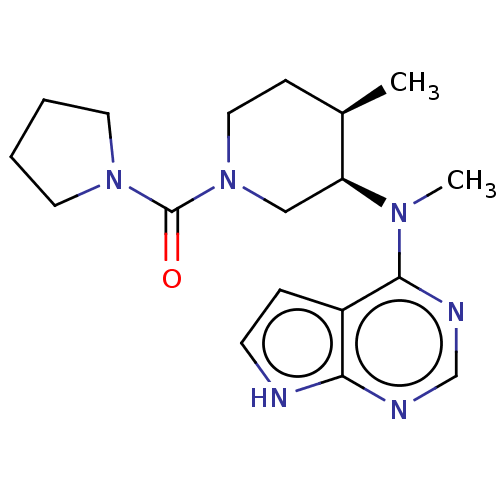
(CHEMBL4068357 | D3RKN_91)Show SMILES C[C@@H]1CCN(C[C@@H]1N(C)c1ncnc2[nH]ccc12)C(=O)N1CCCC1 |r| Show InChI InChI=1S/C18H26N6O/c1-13-6-10-24(18(25)23-8-3-4-9-23)11-15(13)22(2)17-14-5-7-19-16(14)20-12-21-17/h5,7,12-13,15H,3-4,6,8-11H2,1-2H3,(H,19,20,21)/t13-,15+/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 2.80 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of human JAK3 using FITC-KGGEEEEYFELVKK as substrate by caliper microfluidic mobility shift assay |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.0c00093
BindingDB Entry DOI: 10.7270/Q2MC93N5 |
More data for this
Ligand-Target Pair | |
B-cell lymphoma 6 protein
(Homo sapiens) | BDBM50546198

(CHEMBL4795171)Show SMILES CNC(=O)COc1cc2cc(Nc3nc(ncc3Cl)N3C[C@@H](C)C[C@@H](C)C3)cc(OC)c2n(C)c1=O |r| | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of BCOR peptide binding to BCL6 BTB domain (5 to 129 residues) C8Q/C67R/C84N triple mutant (unknown origin) expressed in Escherichia coli ... |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.0c00093
BindingDB Entry DOI: 10.7270/Q2MC93N5 |
More data for this
Ligand-Target Pair | |
B-cell lymphoma 6 protein
(Homo sapiens) | BDBM50546197
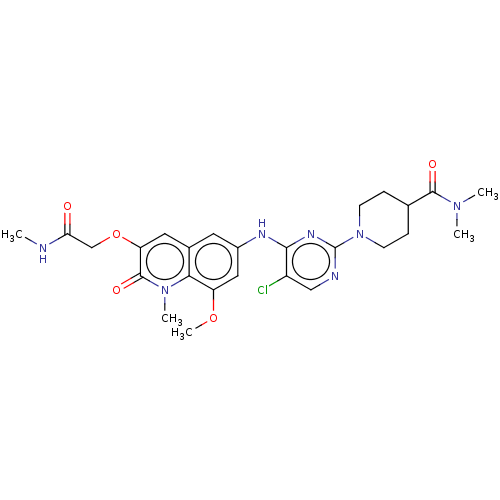
(CHEMBL4755229)Show SMILES CNC(=O)COc1cc2cc(Nc3nc(ncc3Cl)N3CCC(CC3)C(=O)N(C)C)cc(OC)c2n(C)c1=O | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of BCOR peptide binding to BCL6 BTB domain (5 to 129 residues) C8Q/C67R/C84N triple mutant (unknown origin) expressed in Escherichia coli ... |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.0c00093
BindingDB Entry DOI: 10.7270/Q2MC93N5 |
More data for this
Ligand-Target Pair | |
Leucine-rich repeat serine/threonine-protein kinase 2
(Homo sapiens (Human)) | BDBM482157

(BDBM50379529 | LRRK2-IN-1 | US11370796, Compound L...)Show SMILES COc1cc(ccc1Nc1ncc2N(C)C(=O)c3ccccc3N(C)c2n1)C(=O)N1CCC(CC1)N1CCN(C)CC1 Show InChI InChI=1S/C31H38N8O3/c1-35-15-17-38(18-16-35)22-11-13-39(14-12-22)29(40)21-9-10-24(27(19-21)42-4)33-31-32-20-26-28(34-31)36(2)25-8-6-5-7-23(25)30(41)37(26)3/h5-10,19-20,22H,11-18H2,1-4H3,(H,32,33,34) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 6 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of GST-LRRK2 (1326 to 2527 residues) G2019S mutant (unknown origin) expressed in HEK293 cells incubated for 15 mins by cerenkov counting m... |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.0c00093
BindingDB Entry DOI: 10.7270/Q2MC93N5 |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase JAK2
(Homo sapiens (Human)) | BDBM50234898

(CHEMBL4068357 | D3RKN_91)Show SMILES C[C@@H]1CCN(C[C@@H]1N(C)c1ncnc2[nH]ccc12)C(=O)N1CCCC1 |r| Show InChI InChI=1S/C18H26N6O/c1-13-6-10-24(18(25)23-8-3-4-9-23)11-15(13)22(2)17-14-5-7-19-16(14)20-12-21-17/h5,7,12-13,15H,3-4,6,8-11H2,1-2H3,(H,19,20,21)/t13-,15+/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
Article
PubMed
| n/a | n/a | 7.10 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of human JAK2 using FITC-KGGEEEEYFELVKK as substrate by caliper microfluidic mobility shift assay |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.0c00093
BindingDB Entry DOI: 10.7270/Q2MC93N5 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Tyrosine-protein kinase JAK1
(Homo sapiens (Human)) | BDBM50234898

(CHEMBL4068357 | D3RKN_91)Show SMILES C[C@@H]1CCN(C[C@@H]1N(C)c1ncnc2[nH]ccc12)C(=O)N1CCCC1 |r| Show InChI InChI=1S/C18H26N6O/c1-13-6-10-24(18(25)23-8-3-4-9-23)11-15(13)22(2)17-14-5-7-19-16(14)20-12-21-17/h5,7,12-13,15H,3-4,6,8-11H2,1-2H3,(H,19,20,21)/t13-,15+/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 7.5 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of human JAK1 using 5FAM-KKSRGDYMTMQID as substrate by caliper microfluidic mobility shift assay |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.0c00093
BindingDB Entry DOI: 10.7270/Q2MC93N5 |
More data for this
Ligand-Target Pair | |
Non-receptor tyrosine-protein kinase TYK2
(Homo sapiens (Human)) | BDBM50234898

(CHEMBL4068357 | D3RKN_91)Show SMILES C[C@@H]1CCN(C[C@@H]1N(C)c1ncnc2[nH]ccc12)C(=O)N1CCCC1 |r| Show InChI InChI=1S/C18H26N6O/c1-13-6-10-24(18(25)23-8-3-4-9-23)11-15(13)22(2)17-14-5-7-19-16(14)20-12-21-17/h5,7,12-13,15H,3-4,6,8-11H2,1-2H3,(H,19,20,21)/t13-,15+/m1/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 128 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of human TYK2 using 5FAM-KKSRGDYMTMQID as substrate by caliper microfluidic mobility shift assay |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.0c00093
BindingDB Entry DOI: 10.7270/Q2MC93N5 |
More data for this
Ligand-Target Pair | |
Androgen receptor
(Homo sapiens (Human)) | BDBM50432188

(CHEMBL2346976)Show SMILES CC(=O)N1CCN(CCOc2ccc(cc2)C2CCN(CC2)C2=Nn3c(CC2)nnc3C(F)(F)F)CC1 |t:24| Show InChI InChI=1S/C25H32F3N7O2/c1-18(36)33-14-12-32(13-15-33)16-17-37-21-4-2-19(3-5-21)20-8-10-34(11-9-20)23-7-6-22-29-30-24(25(26,27)28)35(22)31-23/h2-5,20H,6-17H2,1H3 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1.78E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Induction of androgen receptor degradation in human LNCaP cells |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.0c00093
BindingDB Entry DOI: 10.7270/Q2MC93N5 |
More data for this
Ligand-Target Pair | |
B-cell lymphoma 6 protein
(Homo sapiens) | BDBM50546199
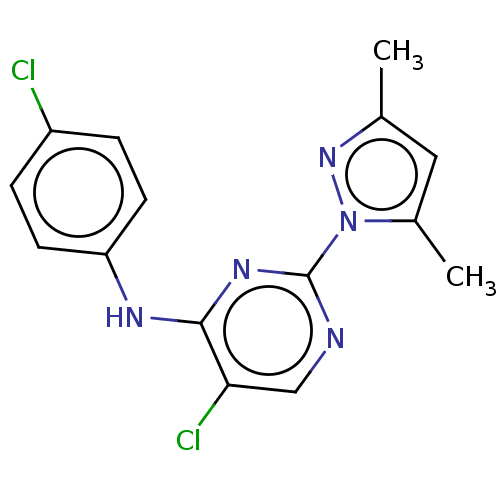
(CHEMBL4751268) | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
PDB
UniChem
| PDB
Article
PubMed
| n/a | n/a | 1.80E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of BCL6 BTB domain (5 to 129 residues) C8Q/C67R/C84N triple mutant (unknown origin) expressed in Escherichia coli BL21(DE3) cells using 5-... |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.0c00093
BindingDB Entry DOI: 10.7270/Q2MC93N5 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Baculoviral IAP repeat-containing protein 2
(Homo sapiens (Human)) | BDBM50546196
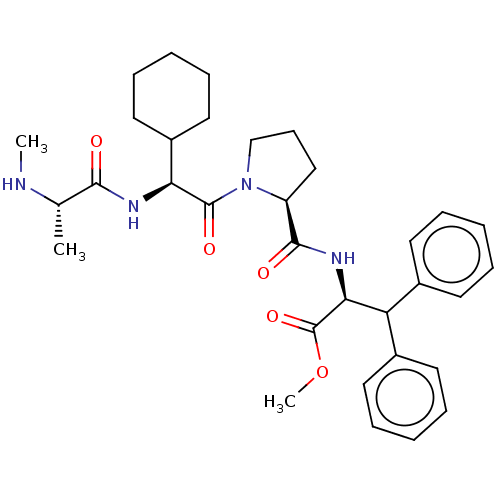
(CHEBI:47922 | CHEMBL2048500)Show SMILES CN[C@@H](C)C(=O)N[C@@H](C1CCCCC1)C(=O)N1CCC[C@H]1C(=O)N[C@@H](C(c1ccccc1)c1ccccc1)C(=O)OC |r| | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | n/a | 6 | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Binding affinity to c-IAP1-BIR3 (unknown origin) (124 to 240 residues) expressed in Escherichia coli BL21-Gold (DE3) by surface plasmon resonance ass... |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.0c00093
BindingDB Entry DOI: 10.7270/Q2MC93N5 |
More data for this
Ligand-Target Pair | |
E3 ubiquitin-protein ligase XIAP
(Homo sapiens (Human)) | BDBM50546196

(CHEBI:47922 | CHEMBL2048500)Show SMILES CN[C@@H](C)C(=O)N[C@@H](C1CCCCC1)C(=O)N1CCC[C@H]1C(=O)N[C@@H](C(c1ccccc1)c1ccccc1)C(=O)OC |r| | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | n/a | 16 | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Binding affinity to XIAP-BIR3 (unknown origin) (124 to 240 residues) expressed in Escherichia coli BL21-Gold (DE3) by surface plasmon resonance assay |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.0c00093
BindingDB Entry DOI: 10.7270/Q2MC93N5 |
More data for this
Ligand-Target Pair | |
Androgen receptor
(Homo sapiens (Human)) | BDBM50529667

(CHEMBL4444904 | US10806720, Compound 11 | US112305...)Show SMILES C[C@](O)(Cn1ccc2cc(F)ccc12)C(=O)Nc1ccc(C#N)c(c1)C(F)(F)F |r| Show InChI InChI=1S/C20H15F4N3O2/c1-19(29,11-27-7-6-12-8-14(21)3-5-17(12)27)18(28)26-15-4-2-13(10-25)16(9-15)20(22,23)24/h2-9,29H,11H2,1H3,(H,26,28)/t19-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | n/a | 1.30E+3 | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of androgen receptor AF1 domain (141 to 486 residues) (unknown origin) incubated for 30 mins by steady-state fluorescence emission spectro... |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.0c00093
BindingDB Entry DOI: 10.7270/Q2MC93N5 |
More data for this
Ligand-Target Pair | |
Histone-lysine N-methyltransferase EZH2
(Homo sapiens (Human)) | BDBM50546194

(CHEMBL4755961)Show SMILES [#6]\[#6](-[#6])=[#6]\[#6]-[#6]\[#6](-[#6])=[#6]\[#6]-c1c(-[#8])c(-[#6]\[#6]=[#6](\[#6])-[#6])c2-[#8]C34[#6]5-[#6]-[#6](-[#6]=[#6]3-[#6](=O)-c2c1-[#8])-[#6](=O)C4([#6]\[#6]=[#6](/[#6])-[#6](-[#8])=O)[#8]C5([#6])[#6] |c:25,TLB:33:32:26.25:22.23,THB:43:22:26.25:32.34,35:34:26.25:22.23,27:26:32.34:22.23| | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | n/a | 182 | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Binding affinity to EZH2 (unknown origin) |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.0c00093
BindingDB Entry DOI: 10.7270/Q2MC93N5 |
More data for this
Ligand-Target Pair | |
Histone-lysine N-methyltransferase EZH2
(Homo sapiens (Human)) | BDBM50546195

(CHEMBL4777537)Show SMILES [#6]-[#6]-[#8]-[#6]-[#6]-[#7]-[#6](=O)-[#6](\[#6])=[#6]\[#6]C12[#8]C([#6])([#6])[#6]3-[#6]-[#6](-[#6]=[#6]4-[#6](=O)-c5c(-[#8])c(-[#6]\[#6]=[#6](/[#6])-[#6]-[#6]\[#6]=[#6](\[#6])-[#6])c(-[#8])c(-[#6]\[#6]=[#6](\[#6])-[#6])c5-[#8]C134)-[#6]2=O |t:20,TLB:50:49:21.20:17.18,THB:14:17:21.20:49.12,11:12:21.20:17.18,22:21:49.12:17.18| | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | n/a | 116 | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Binding affinity to EZH2 (unknown origin) |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.0c00093
BindingDB Entry DOI: 10.7270/Q2MC93N5 |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data