

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Indoleamine 2,3-dioxygenase 1 (Homo sapiens (Human)) | BDBM50126143 (Epacadostat | INCB-024360) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid PDB UniChem Similars | PDB Article PubMed | n/a | n/a | 30 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of recombinant human IDO1 expressed in Escherichia coli EC538 assessed as reduction in N-formylkynurenine formation using L-tryptophan as ... | Citation and Details Article DOI: 10.1016/j.bmc.2021.116160 BindingDB Entry DOI: 10.7270/Q2DF6W0V | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Tryptophan 2,3-dioxygenase (Homo sapiens (Human)) | BDBM50126144 (CHEMBL3629569 | US10155972, Compound NewLink 1 | U...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank GoogleScholar AffyNet | Purchase CHEMBL MCE KEGG PC cid PC sid PDB UniChem Similars | Article PubMed | n/a | n/a | 30 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of recombinant human TDO2 expressed in Escherichia coli BL21 (DE3) assessed as reduction in N-formylkynurenine formation using L-tryptopha... | Citation and Details Article DOI: 10.1016/j.bmc.2021.116160 BindingDB Entry DOI: 10.7270/Q2DF6W0V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Indoleamine 2,3-dioxygenase 1 (Homo sapiens (Human)) | BDBM50126143 (Epacadostat | INCB-024360) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid PDB UniChem Similars | PDB Article PubMed | n/a | n/a | 40 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of recombinant human IDO1 expressed in Escherichia coli EC538 assessed as reduction in N-formylkynurenine formation using L-tryptophan as ... | Citation and Details Article DOI: 10.1016/j.bmc.2021.116160 BindingDB Entry DOI: 10.7270/Q2DF6W0V | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Tryptophan 2,3-dioxygenase (Rattus norvegicus) | BDBM50289137 (6-Fluoro-3-((E)-2-pyridin-3-yl-vinyl)-1H-indole | ...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 50 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of TDO2 in rat liver homogenate assessed as reduction in N-formylkynurenine formation using L-tryptophan as substrate incubated for 1 hr | Citation and Details Article DOI: 10.1016/j.bmc.2021.116160 BindingDB Entry DOI: 10.7270/Q2DF6W0V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Indoleamine 2,3-dioxygenase 1 (Homo sapiens (Human)) | BDBM50126144 (CHEMBL3629569 | US10155972, Compound NewLink 1 | U...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE KEGG PC cid PC sid PDB UniChem Similars | PDB Article PubMed | n/a | n/a | 50 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of recombinant human IDO1 assessed as reduction in N-formylkynurenine formation using L-tryptophan as substrate incubated for 45 mins by m... | Citation and Details Article DOI: 10.1016/j.bmc.2021.116160 BindingDB Entry DOI: 10.7270/Q2DF6W0V | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Indoleamine 2,3-dioxygenase 1 (Homo sapiens (Human)) | BDBM50235921 (CHEMBL584991) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 60 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of recombinant human IDO1 expressed in Escherichia coli EC538 assessed as reduction in N-formylkynurenine formation using L-tryptophan as ... | Citation and Details Article DOI: 10.1016/j.bmc.2021.116160 BindingDB Entry DOI: 10.7270/Q2DF6W0V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Indoleamine 2,3-dioxygenase 1 (Homo sapiens (Human)) | BDBM50126144 (CHEMBL3629569 | US10155972, Compound NewLink 1 | U...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE KEGG PC cid PC sid PDB UniChem Similars | PDB Article PubMed | n/a | n/a | 80 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of recombinant human IDO1 expressed in Escherichia coli EC538 assessed as reduction in N-formylkynurenine formation using L-tryptophan as ... | Citation and Details Article DOI: 10.1016/j.bmc.2021.116160 BindingDB Entry DOI: 10.7270/Q2DF6W0V | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Indoleamine 2,3-dioxygenase 1 (Homo sapiens (Human)) | BDBM50126144 (CHEMBL3629569 | US10155972, Compound NewLink 1 | U...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE KEGG PC cid PC sid PDB UniChem Similars | PDB Article PubMed | n/a | n/a | 110 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of recombinant human IDO1 expressed in Escherichia coli EC538 assessed as reduction in N-formylkynurenine formation using L-tryptophan as ... | Citation and Details Article DOI: 10.1016/j.bmc.2021.116160 BindingDB Entry DOI: 10.7270/Q2DF6W0V | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Tryptophan 2,3-dioxygenase (Homo sapiens (Human)) | BDBM50289137 (6-Fluoro-3-((E)-2-pyridin-3-yl-vinyl)-1H-indole | ...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 110 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of recombinant human TDO2 expressed in Escherichia coli BL21 (DE3) assessed as reduction in N-formylkynurenine formation using L-tryptopha... | Citation and Details Article DOI: 10.1016/j.bmc.2021.116160 BindingDB Entry DOI: 10.7270/Q2DF6W0V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Indoleamine 2,3-dioxygenase 1 (Homo sapiens (Human)) | BDBM50235921 (CHEMBL584991) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 130 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of recombinant human IDO1 expressed in Escherichia coli EC538 assessed as reduction in N-formylkynurenine formation using L-tryptophan as ... | Citation and Details Article DOI: 10.1016/j.bmc.2021.116160 BindingDB Entry DOI: 10.7270/Q2DF6W0V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tryptophan 2,3-dioxygenase (Homo sapiens (Human)) | BDBM50126144 (CHEMBL3629569 | US10155972, Compound NewLink 1 | U...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank GoogleScholar AffyNet | Purchase CHEMBL MCE KEGG PC cid PC sid PDB UniChem Similars | Article PubMed | n/a | n/a | 150 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of recombinant human TDO2 expressed in Escherichia coli BL21 (DE3) assessed as reduction in N-formylkynurenine formation using L-tryptopha... | Citation and Details Article DOI: 10.1016/j.bmc.2021.116160 BindingDB Entry DOI: 10.7270/Q2DF6W0V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tryptophan 2,3-dioxygenase (Homo sapiens (Human)) | BDBM50289137 (6-Fluoro-3-((E)-2-pyridin-3-yl-vinyl)-1H-indole | ...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 310 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of recombinant human TDO2 expressed in Escherichia coli BL21 (DE3) assessed as reduction in N-formylkynurenine formation using L-tryptopha... | Citation and Details Article DOI: 10.1016/j.bmc.2021.116160 BindingDB Entry DOI: 10.7270/Q2DF6W0V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Indoleamine 2,3-dioxygenase 1 (Homo sapiens (Human)) | BDBM50013793 (CHEMBL3192687) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem | Article PubMed | n/a | n/a | 1.39E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of recombinant human IDO1 expressed in Escherichia coli EC538 assessed as reduction in N-formylkynurenine formation using L-tryptophan as ... | Citation and Details Article DOI: 10.1016/j.bmc.2021.116160 BindingDB Entry DOI: 10.7270/Q2DF6W0V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Indoleamine 2,3-dioxygenase 1 (Homo sapiens (Human)) | BDBM50568051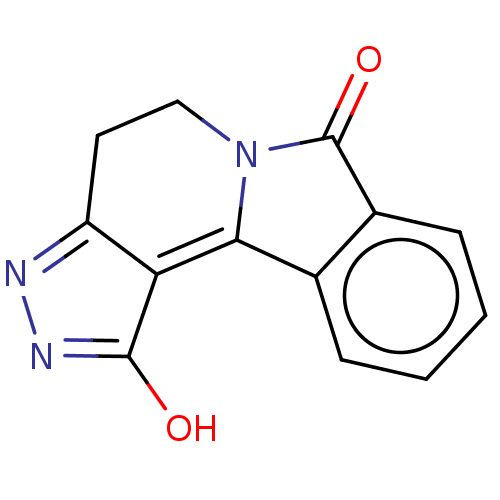 (CHEMBL4092918) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 1.72E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of recombinant human IDO1 expressed in Escherichia coli EC538 assessed as reduction in N-formylkynurenine formation using L-tryptophan as ... | Citation and Details Article DOI: 10.1016/j.bmc.2021.116160 BindingDB Entry DOI: 10.7270/Q2DF6W0V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tryptophan 2,3-dioxygenase (Homo sapiens (Human)) | BDBM50126143 (Epacadostat | INCB-024360) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank GoogleScholar AffyNet | Purchase MCE PC cid PC sid PDB UniChem Similars | Article PubMed | n/a | n/a | 2.12E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of recombinant human TDO2 expressed in Escherichia coli BL21 (DE3) assessed as reduction in N-formylkynurenine formation using L-tryptopha... | Citation and Details Article DOI: 10.1016/j.bmc.2021.116160 BindingDB Entry DOI: 10.7270/Q2DF6W0V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Indoleamine 2,3-dioxygenase 1 (Homo sapiens (Human)) | BDBM50568048 (CHEMBL1981840) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 2.79E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of recombinant human IDO1 expressed in Escherichia coli EC538 assessed as reduction in N-formylkynurenine formation using L-tryptophan as ... | Citation and Details Article DOI: 10.1016/j.bmc.2021.116160 BindingDB Entry DOI: 10.7270/Q2DF6W0V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tryptophan 2,3-dioxygenase (Homo sapiens (Human)) | BDBM50126143 (Epacadostat | INCB-024360) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank GoogleScholar AffyNet | Purchase MCE PC cid PC sid PDB UniChem Similars | Article PubMed | n/a | n/a | 3.43E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of recombinant human TDO2 expressed in Escherichia coli BL21 (DE3) assessed as reduction in N-formylkynurenine formation using L-tryptopha... | Citation and Details Article DOI: 10.1016/j.bmc.2021.116160 BindingDB Entry DOI: 10.7270/Q2DF6W0V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tryptophan 2,3-dioxygenase (Homo sapiens (Human)) | BDBM50235921 (CHEMBL584991) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 3.47E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of recombinant human TDO2 expressed in Escherichia coli BL21 (DE3) assessed as reduction in N-formylkynurenine formation using L-tryptopha... | Citation and Details Article DOI: 10.1016/j.bmc.2021.116160 BindingDB Entry DOI: 10.7270/Q2DF6W0V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tryptophan 2,3-dioxygenase (Homo sapiens (Human)) | BDBM50235921 (CHEMBL584991) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 3.62E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of recombinant human TDO2 expressed in Escherichia coli BL21 (DE3) assessed as reduction in N-formylkynurenine formation using L-tryptopha... | Citation and Details Article DOI: 10.1016/j.bmc.2021.116160 BindingDB Entry DOI: 10.7270/Q2DF6W0V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Indoleamine 2,3-dioxygenase 1 (Homo sapiens (Human)) | BDBM50568050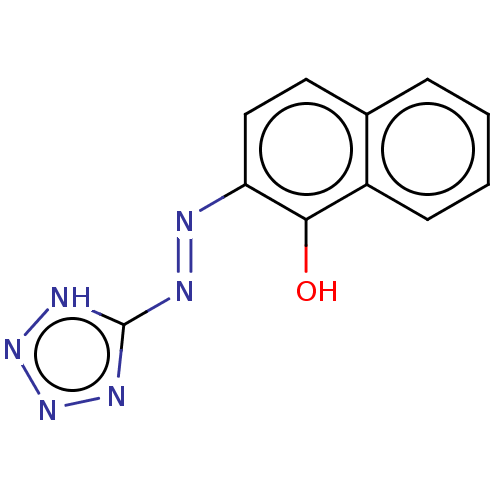 (CHEMBL4296947) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 3.78E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of recombinant human IDO1 expressed in Escherichia coli EC538 assessed as reduction in N-formylkynurenine formation using L-tryptophan as ... | Citation and Details Article DOI: 10.1016/j.bmc.2021.116160 BindingDB Entry DOI: 10.7270/Q2DF6W0V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tryptophan 2,3-dioxygenase (Homo sapiens (Human)) | BDBM50568051 (CHEMBL4092918) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 4.35E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of recombinant human TDO2 expressed in Escherichia coli BL21 (DE3) assessed as reduction in N-formylkynurenine formation using L-tryptopha... | Citation and Details Article DOI: 10.1016/j.bmc.2021.116160 BindingDB Entry DOI: 10.7270/Q2DF6W0V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Indoleamine 2,3-dioxygenase 1 (Homo sapiens (Human)) | BDBM50568049 (CHEMBL1487639) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 4.37E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of recombinant human IDO1 expressed in Escherichia coli EC538 assessed as reduction in N-formylkynurenine formation using L-tryptophan as ... | Citation and Details Article DOI: 10.1016/j.bmc.2021.116160 BindingDB Entry DOI: 10.7270/Q2DF6W0V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Indoleamine 2,3-dioxygenase 1 (Homo sapiens (Human)) | BDBM50071058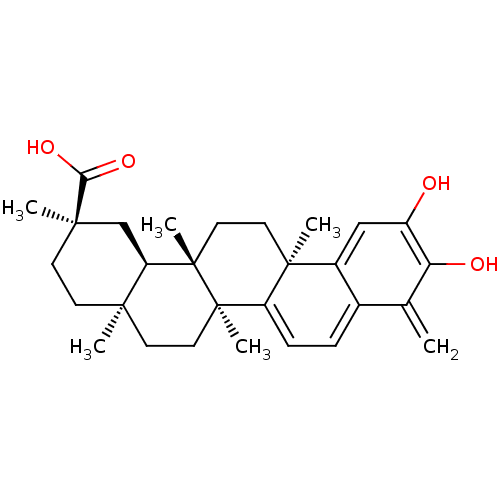 ((2R,4aS,6aS,12bR,14aS,14bR)-10-Hydroxy-2,4a,6a,9,1...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 8.41E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of recombinant human IDO1 expressed in Escherichia coli EC538 assessed as reduction in N-formylkynurenine formation using L-tryptophan as ... | Citation and Details Article DOI: 10.1016/j.bmc.2021.116160 BindingDB Entry DOI: 10.7270/Q2DF6W0V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tryptophan 2,3-dioxygenase (Homo sapiens (Human)) | BDBM50568044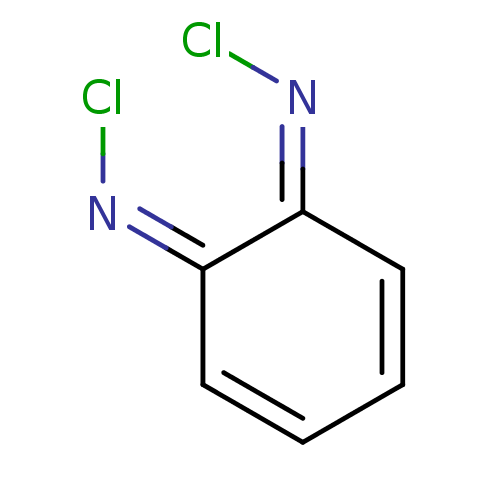 (CHEMBL4296926) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 8.43E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of recombinant human TDO2 expressed in Escherichia coli BL21 (DE3) assessed as reduction in N-formylkynurenine formation using L-tryptopha... | Citation and Details Article DOI: 10.1016/j.bmc.2021.116160 BindingDB Entry DOI: 10.7270/Q2DF6W0V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Indoleamine 2,3-dioxygenase 1 (Homo sapiens (Human)) | BDBM50568044 (CHEMBL4296926) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 9.17E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of recombinant human IDO1 expressed in Escherichia coli EC538 assessed as reduction in N-formylkynurenine formation using L-tryptophan as ... | Citation and Details Article DOI: 10.1016/j.bmc.2021.116160 BindingDB Entry DOI: 10.7270/Q2DF6W0V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Indoleamine 2,3-dioxygenase 1 (Homo sapiens (Human)) | BDBM50425026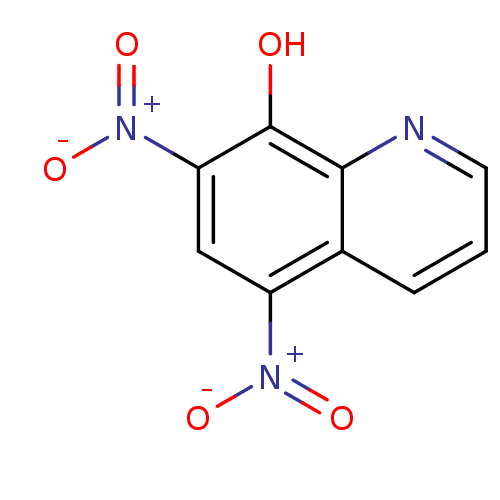 (CHEMBL241898) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | 1.11E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of recombinant human IDO1 expressed in Escherichia coli EC538 assessed as reduction in N-formylkynurenine formation using L-tryptophan as ... | Citation and Details Article DOI: 10.1016/j.bmc.2021.116160 BindingDB Entry DOI: 10.7270/Q2DF6W0V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tryptophan 2,3-dioxygenase (Homo sapiens (Human)) | BDBM50013793 (CHEMBL3192687) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem | Article PubMed | n/a | n/a | 1.13E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of recombinant human TDO2 expressed in Escherichia coli BL21 (DE3) assessed as reduction in N-formylkynurenine formation using L-tryptopha... | Citation and Details Article DOI: 10.1016/j.bmc.2021.116160 BindingDB Entry DOI: 10.7270/Q2DF6W0V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Indoleamine 2,3-dioxygenase 1 (Homo sapiens (Human)) | BDBM58106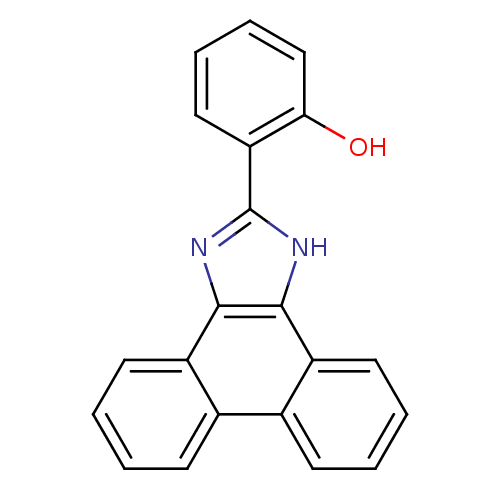 (6-(1,3-dihydrophenanthr[9,10-d]imidazol-2-ylidene)...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 1.18E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of recombinant human IDO1 expressed in Escherichia coli EC538 assessed as reduction in N-formylkynurenine formation using L-tryptophan as ... | Citation and Details Article DOI: 10.1016/j.bmc.2021.116160 BindingDB Entry DOI: 10.7270/Q2DF6W0V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tryptophan 2,3-dioxygenase (Homo sapiens (Human)) | BDBM58106 (6-(1,3-dihydrophenanthr[9,10-d]imidazol-2-ylidene)...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank GoogleScholar AffyNet | Purchase PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 1.23E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of recombinant human TDO2 expressed in Escherichia coli BL21 (DE3) assessed as reduction in N-formylkynurenine formation using L-tryptopha... | Citation and Details Article DOI: 10.1016/j.bmc.2021.116160 BindingDB Entry DOI: 10.7270/Q2DF6W0V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Indoleamine 2,3-dioxygenase 1 (Homo sapiens (Human)) | BDBM66065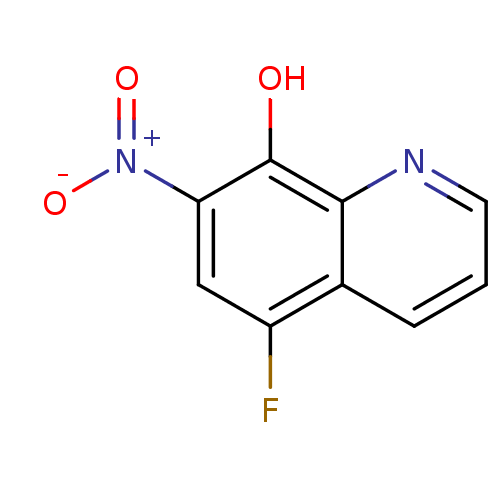 (5-fluoranyl-7-nitro-quinolin-8-ol | 5-fluoro-7-nit...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 1.46E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of recombinant human IDO1 expressed in Escherichia coli EC538 assessed as reduction in N-formylkynurenine formation using L-tryptophan as ... | Citation and Details Article DOI: 10.1016/j.bmc.2021.116160 BindingDB Entry DOI: 10.7270/Q2DF6W0V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tryptophan 2,3-dioxygenase (Homo sapiens (Human)) | BDBM50568045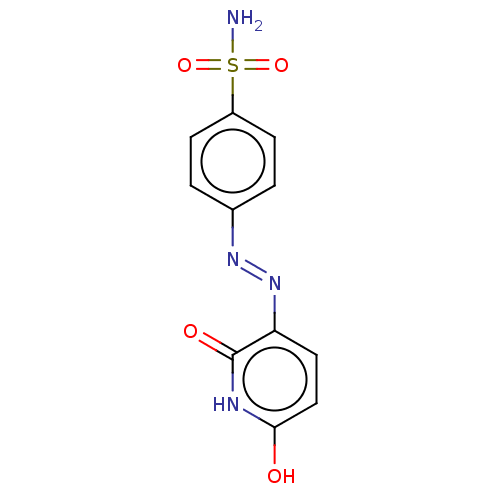 (CHEMBL4296952) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank GoogleScholar AffyNet | PC cid PC sid PDB UniChem | Article PubMed | n/a | n/a | 1.57E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of recombinant human TDO2 expressed in Escherichia coli BL21 (DE3) assessed as reduction in N-formylkynurenine formation using L-tryptopha... | Citation and Details Article DOI: 10.1016/j.bmc.2021.116160 BindingDB Entry DOI: 10.7270/Q2DF6W0V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tryptophan 2,3-dioxygenase (Homo sapiens (Human)) | BDBM50568046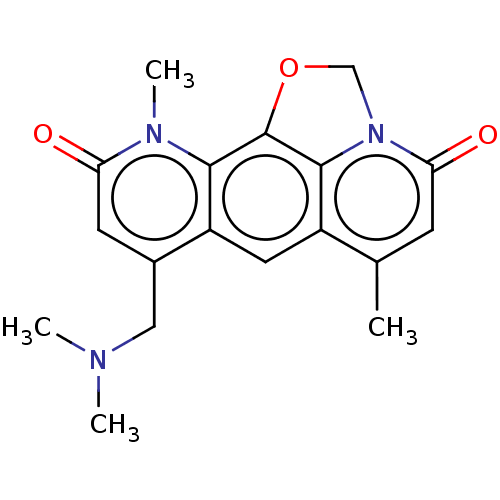 (CHEMBL2000037) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 1.77E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of recombinant human TDO2 expressed in Escherichia coli BL21 (DE3) assessed as reduction in N-formylkynurenine formation using L-tryptopha... | Citation and Details Article DOI: 10.1016/j.bmc.2021.116160 BindingDB Entry DOI: 10.7270/Q2DF6W0V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Indoleamine 2,3-dioxygenase 1 (Homo sapiens (Human)) | BDBM50568045 (CHEMBL4296952) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid PDB UniChem | Article PubMed | n/a | n/a | 1.81E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of recombinant human IDO1 expressed in Escherichia coli EC538 assessed as reduction in N-formylkynurenine formation using L-tryptophan as ... | Citation and Details Article DOI: 10.1016/j.bmc.2021.116160 BindingDB Entry DOI: 10.7270/Q2DF6W0V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tryptophan 2,3-dioxygenase (Homo sapiens (Human)) | BDBM50568050 (CHEMBL4296947) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 2.23E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of recombinant human TDO2 expressed in Escherichia coli BL21 (DE3) assessed as reduction in N-formylkynurenine formation using L-tryptopha... | Citation and Details Article DOI: 10.1016/j.bmc.2021.116160 BindingDB Entry DOI: 10.7270/Q2DF6W0V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tryptophan 2,3-dioxygenase (Homo sapiens (Human)) | BDBM50425026 (CHEMBL241898) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | 2.27E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of recombinant human TDO2 expressed in Escherichia coli BL21 (DE3) assessed as reduction in N-formylkynurenine formation using L-tryptopha... | Citation and Details Article DOI: 10.1016/j.bmc.2021.116160 BindingDB Entry DOI: 10.7270/Q2DF6W0V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Indoleamine 2,3-dioxygenase 1 (Homo sapiens (Human)) | BDBM91466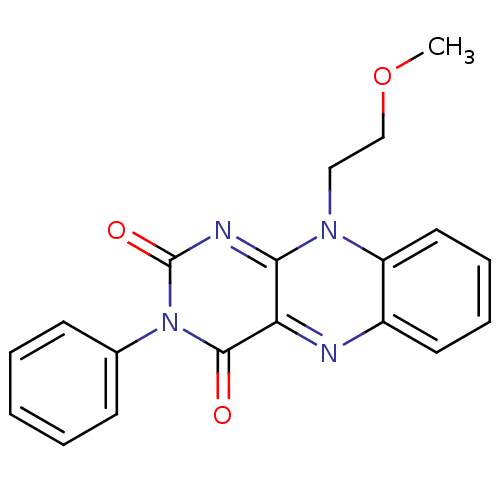 (10-(2-methoxyethyl)-3-phenyl-benzo[g]pteridine-2,4...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | MCE PC cid PC sid UniChem | Article PubMed | n/a | n/a | 2.38E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of recombinant human IDO1 expressed in Escherichia coli EC538 assessed as reduction in N-formylkynurenine formation using L-tryptophan as ... | Citation and Details Article DOI: 10.1016/j.bmc.2021.116160 BindingDB Entry DOI: 10.7270/Q2DF6W0V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Indoleamine 2,3-dioxygenase 1 (Homo sapiens (Human)) | BDBM50568046 (CHEMBL2000037) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 2.70E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of recombinant human IDO1 expressed in Escherichia coli EC538 assessed as reduction in N-formylkynurenine formation using L-tryptophan as ... | Citation and Details Article DOI: 10.1016/j.bmc.2021.116160 BindingDB Entry DOI: 10.7270/Q2DF6W0V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tryptophan 2,3-dioxygenase (Homo sapiens (Human)) | BDBM66065 (5-fluoranyl-7-nitro-quinolin-8-ol | 5-fluoro-7-nit...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 3.02E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of recombinant human TDO2 expressed in Escherichia coli BL21 (DE3) assessed as reduction in N-formylkynurenine formation using L-tryptopha... | Citation and Details Article DOI: 10.1016/j.bmc.2021.116160 BindingDB Entry DOI: 10.7270/Q2DF6W0V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tryptophan 2,3-dioxygenase (Homo sapiens (Human)) | BDBM91466 (10-(2-methoxyethyl)-3-phenyl-benzo[g]pteridine-2,4...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank GoogleScholar AffyNet | MCE PC cid PC sid UniChem | Article PubMed | n/a | n/a | 3.37E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of recombinant human TDO2 expressed in Escherichia coli BL21 (DE3) assessed as reduction in N-formylkynurenine formation using L-tryptopha... | Citation and Details Article DOI: 10.1016/j.bmc.2021.116160 BindingDB Entry DOI: 10.7270/Q2DF6W0V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tryptophan 2,3-dioxygenase (Homo sapiens (Human)) | BDBM61215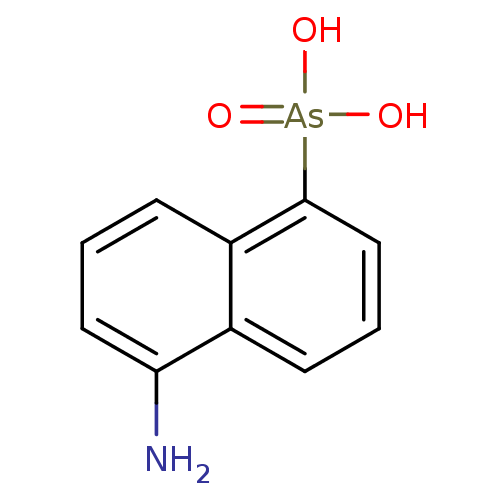 ((5-amino-1-naphthalenyl)arsonic acid | (5-amino-1-...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 3.79E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of recombinant human TDO2 expressed in Escherichia coli BL21 (DE3) assessed as reduction in N-formylkynurenine formation using L-tryptopha... | Citation and Details Article DOI: 10.1016/j.bmc.2021.116160 BindingDB Entry DOI: 10.7270/Q2DF6W0V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tryptophan 2,3-dioxygenase (Homo sapiens (Human)) | BDBM50568047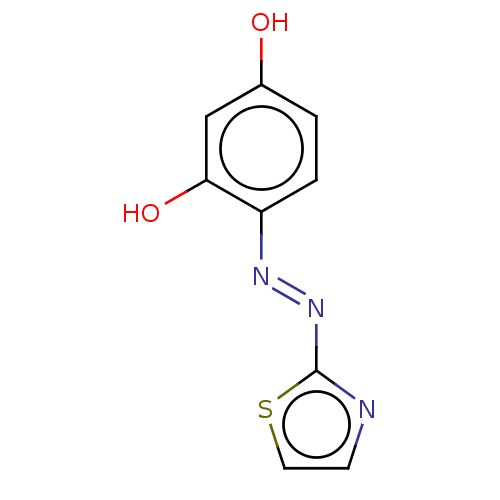 (CHEMBL4296835) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 4.33E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of recombinant human TDO2 expressed in Escherichia coli BL21 (DE3) assessed as reduction in N-formylkynurenine formation using L-tryptopha... | Citation and Details Article DOI: 10.1016/j.bmc.2021.116160 BindingDB Entry DOI: 10.7270/Q2DF6W0V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Indoleamine 2,3-dioxygenase 1 (Homo sapiens (Human)) | BDBM50124967 (5,6,8,5',6',8'-Hexahydroxy-2,3,2',3'-tetramethyl-2...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 4.41E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of recombinant human IDO1 expressed in Escherichia coli EC538 assessed as reduction in N-formylkynurenine formation using L-tryptophan as ... | Citation and Details Article DOI: 10.1016/j.bmc.2021.116160 BindingDB Entry DOI: 10.7270/Q2DF6W0V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Indoleamine 2,3-dioxygenase 1 (Homo sapiens (Human)) | BDBM50568047 (CHEMBL4296835) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 4.50E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of recombinant human IDO1 expressed in Escherichia coli EC538 assessed as reduction in N-formylkynurenine formation using L-tryptophan as ... | Citation and Details Article DOI: 10.1016/j.bmc.2021.116160 BindingDB Entry DOI: 10.7270/Q2DF6W0V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Indoleamine 2,3-dioxygenase 1 (Homo sapiens (Human)) | BDBM61215 ((5-amino-1-naphthalenyl)arsonic acid | (5-amino-1-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 4.57E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of recombinant human IDO1 expressed in Escherichia coli EC538 assessed as reduction in N-formylkynurenine formation using L-tryptophan as ... | Citation and Details Article DOI: 10.1016/j.bmc.2021.116160 BindingDB Entry DOI: 10.7270/Q2DF6W0V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tryptophan 2,3-dioxygenase (Homo sapiens (Human)) | BDBM50124967 (5,6,8,5',6',8'-Hexahydroxy-2,3,2',3'-tetramethyl-2...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 4.86E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of recombinant human TDO2 expressed in Escherichia coli BL21 (DE3) assessed as reduction in N-formylkynurenine formation using L-tryptopha... | Citation and Details Article DOI: 10.1016/j.bmc.2021.116160 BindingDB Entry DOI: 10.7270/Q2DF6W0V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tryptophan 2,3-dioxygenase (Homo sapiens (Human)) | BDBM50071058 ((2R,4aS,6aS,12bR,14aS,14bR)-10-Hydroxy-2,4a,6a,9,1...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of recombinant human TDO2 expressed in Escherichia coli BL21 (DE3) assessed as reduction in N-formylkynurenine formation using L-tryptopha... | Citation and Details Article DOI: 10.1016/j.bmc.2021.116160 BindingDB Entry DOI: 10.7270/Q2DF6W0V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tryptophan 2,3-dioxygenase (Homo sapiens (Human)) | BDBM50568049 (CHEMBL1487639) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of recombinant human TDO2 expressed in Escherichia coli BL21 (DE3) assessed as reduction in N-formylkynurenine formation using L-tryptopha... | Citation and Details Article DOI: 10.1016/j.bmc.2021.116160 BindingDB Entry DOI: 10.7270/Q2DF6W0V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tryptophan 2,3-dioxygenase (Homo sapiens (Human)) | BDBM50568048 (CHEMBL1981840) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of recombinant human TDO2 expressed in Escherichia coli BL21 (DE3) assessed as reduction in N-formylkynurenine formation using L-tryptopha... | Citation and Details Article DOI: 10.1016/j.bmc.2021.116160 BindingDB Entry DOI: 10.7270/Q2DF6W0V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Indoleamine 2,3-dioxygenase 1 (Homo sapiens (Human)) | BDBM50289137 (6-Fluoro-3-((E)-2-pyridin-3-yl-vinyl)-1H-indole | ...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 3.50E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of recombinant human IDO1 expressed in Escherichia coli EC538 assessed as reduction in N-formylkynurenine formation using L-tryptophan as ... | Citation and Details Article DOI: 10.1016/j.bmc.2021.116160 BindingDB Entry DOI: 10.7270/Q2DF6W0V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Indoleamine 2,3-dioxygenase 1 (Homo sapiens (Human)) | BDBM50289137 (6-Fluoro-3-((E)-2-pyridin-3-yl-vinyl)-1H-indole | ...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 4.78E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of recombinant human IDO1 expressed in Escherichia coli EC538 assessed as reduction in N-formylkynurenine formation using L-tryptophan as ... | Citation and Details Article DOI: 10.1016/j.bmc.2021.116160 BindingDB Entry DOI: 10.7270/Q2DF6W0V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||