

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Urokinase-type plasminogen activator (Homo sapiens (Human)) | BDBM233005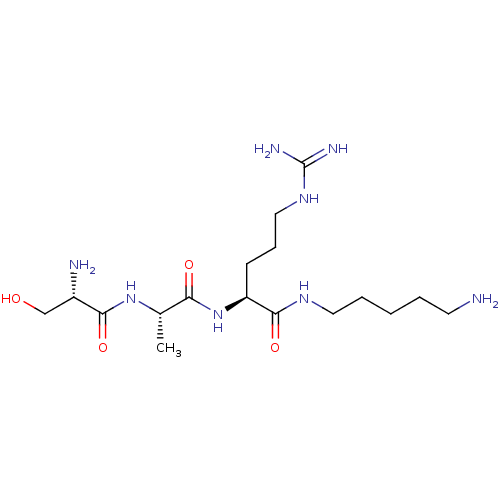 (H-D-Ser-Ala-Arg-NH-(CH2)5-NH2 (4)) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 6.30E+3 | n/a | n/a | n/a | n/a | n/a | n/a | 8.8 | n/a |
Medical University of Bialystok | Assay Description Determination of amidolytic activity was performed as described previously [Okada et al., Chem. Pharm. Bull., 36:1289-1297]. Detailed description of ... | J Enzyme Inhib Med Chem 25: 139-42 (2010) Article DOI: 10.3109/14756360903049042 BindingDB Entry DOI: 10.7270/Q23R0RR3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Urokinase-type plasminogen activator (Homo sapiens (Human)) | BDBM233008 (H-D-Ser-Ala-Arg-NH-(CH2)8-NH2 (7)) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 8.70E+4 | n/a | n/a | n/a | n/a | n/a | n/a | 8.8 | n/a |
Medical University of Bialystok | Assay Description Determination of amidolytic activity was performed as described previously [Okada et al., Chem. Pharm. Bull., 36:1289-1297]. Detailed description of ... | J Enzyme Inhib Med Chem 25: 139-42 (2010) Article DOI: 10.3109/14756360903049042 BindingDB Entry DOI: 10.7270/Q23R0RR3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Urokinase-type plasminogen activator (Homo sapiens (Human)) | BDBM233007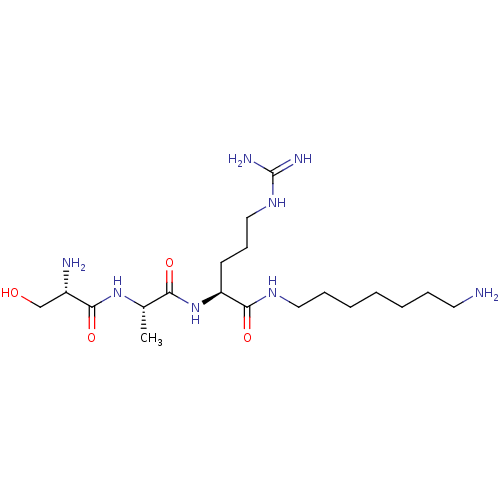 (H-D-Ser-Ala-Arg-NH-(CH2)7-NH2 (6)) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 2.44E+5 | n/a | n/a | n/a | n/a | n/a | n/a | 8.8 | n/a |
Medical University of Bialystok | Assay Description Determination of amidolytic activity was performed as described previously [Okada et al., Chem. Pharm. Bull., 36:1289-1297]. Detailed description of ... | J Enzyme Inhib Med Chem 25: 139-42 (2010) Article DOI: 10.3109/14756360903049042 BindingDB Entry DOI: 10.7270/Q23R0RR3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Urokinase-type plasminogen activator (Homo sapiens (Human)) | BDBM233009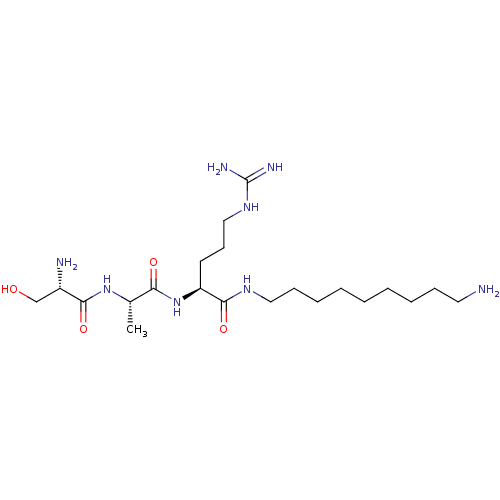 (H-D-Ser-Ala-Arg-NH-(CH2)9-NH2 (8)) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 2.93E+5 | n/a | n/a | n/a | n/a | n/a | n/a | 8.8 | n/a |
Medical University of Bialystok | Assay Description Determination of amidolytic activity was performed as described previously [Okada et al., Chem. Pharm. Bull., 36:1289-1297]. Detailed description of ... | J Enzyme Inhib Med Chem 25: 139-42 (2010) Article DOI: 10.3109/14756360903049042 BindingDB Entry DOI: 10.7270/Q23R0RR3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||