

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Adenosine receptor A2b (Homo sapiens (Human)) | BDBM50241166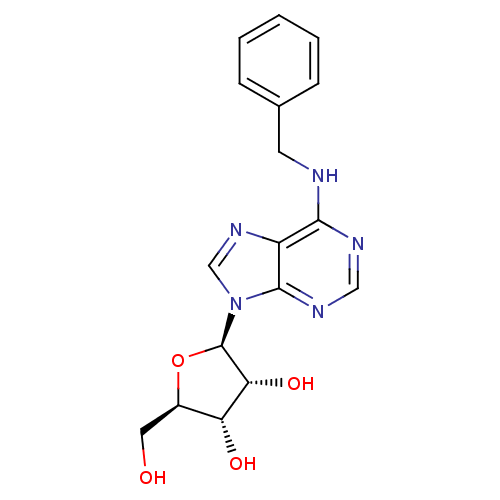 ((2R,3R,4S,5R)-2-(6-(benzylamino)-9H-purin-9-yl)-5-...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | n/a | n/a | 1.59E+4 | n/a | n/a | n/a | n/a |
National Institute of Diabetes and Digestive and Kidney Diseases Curated by ChEMBL | Assay Description Agonist activity at human adenosine A2B receptor expressed in CHO cells assessed as stimulation of adenylate cyclase | J Med Chem 51: 2088-99 (2008) Article DOI: 10.1021/jm701442d BindingDB Entry DOI: 10.7270/Q2R49RNV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A2b (Homo sapiens (Human)) | BDBM50205577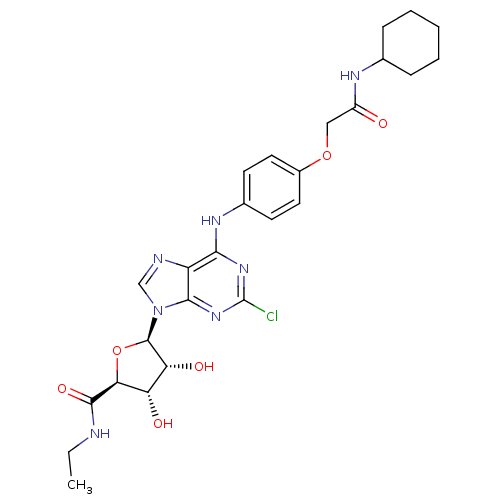 ((2S,3S,4R,5R)-5-(2-chloro-6-(4-(2-(cyclohexylamino...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 3.31E+6 | n/a | n/a | n/a | n/a |
National Institute of Diabetes and Digestive and Kidney Diseases Curated by ChEMBL | Assay Description Agonist activity at human adenosine A2B receptor expressed in CHO cells assessed as stimulation of adenylate cyclase | J Med Chem 51: 2088-99 (2008) Article DOI: 10.1021/jm701442d BindingDB Entry DOI: 10.7270/Q2R49RNV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A2b (Homo sapiens (Human)) | BDBM50205578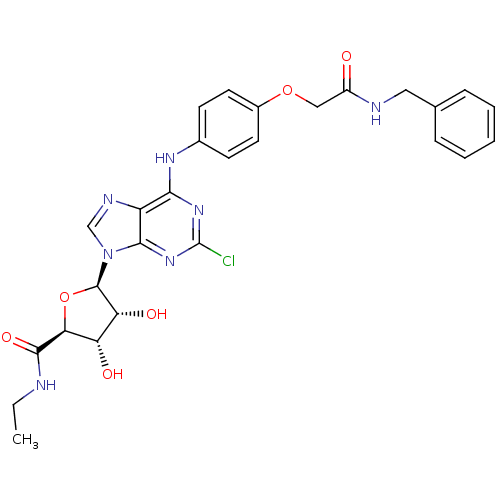 ((2S,3S,4R,5R)-5-(6-(4-(2-(benzylamino)-2-oxoethoxy...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 5.71E+6 | n/a | n/a | n/a | n/a |
National Institute of Diabetes and Digestive and Kidney Diseases Curated by ChEMBL | Assay Description Agonist activity at human adenosine A2B receptor expressed in CHO cells assessed as stimulation of adenylate cyclase | J Med Chem 51: 2088-99 (2008) Article DOI: 10.1021/jm701442d BindingDB Entry DOI: 10.7270/Q2R49RNV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A2b (Homo sapiens (Human)) | BDBM50208782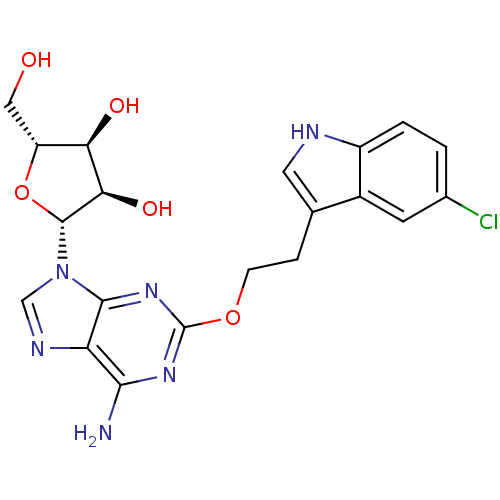 ((2R,3R,4S,5R)-2-(6-amino-2-(2-(5-chloro-1H-indol-3...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 4.58E+5 | n/a | n/a | n/a | n/a |
National Institute of Diabetes and Digestive and Kidney Diseases Curated by ChEMBL | Assay Description Agonist activity at human adenosine A2B receptor expressed in CHO cells assessed as stimulation of adenylate cyclase | J Med Chem 51: 2088-99 (2008) Article DOI: 10.1021/jm701442d BindingDB Entry DOI: 10.7270/Q2R49RNV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A2b (Homo sapiens (Human)) | BDBM50003262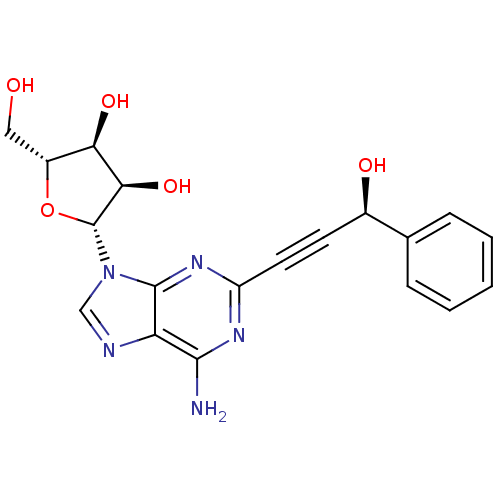 (CHEMBL262363) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 1.09E+6 | n/a | n/a | n/a | n/a |
National Institute of Diabetes and Digestive and Kidney Diseases Curated by ChEMBL | Assay Description Agonist activity at human adenosine A2B receptor expressed in CHO cells assessed as stimulation of adenylate cyclase | J Med Chem 51: 2088-99 (2008) Article DOI: 10.1021/jm701442d BindingDB Entry DOI: 10.7270/Q2R49RNV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A2b (Homo sapiens (Human)) | BDBM50003275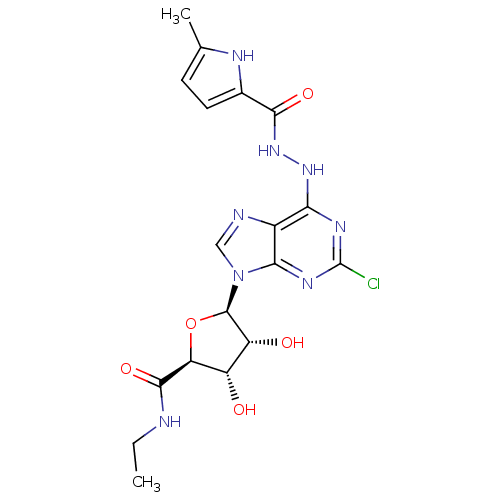 (CHEMBL259297) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | n/a | n/a | 5.71E+6 | n/a | n/a | n/a | n/a |
National Institute of Diabetes and Digestive and Kidney Diseases Curated by ChEMBL | Assay Description Agonist activity at human adenosine A2B receptor expressed in CHO cells assessed as stimulation of adenylate cyclase | J Med Chem 51: 2088-99 (2008) Article DOI: 10.1021/jm701442d BindingDB Entry DOI: 10.7270/Q2R49RNV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A2b (Homo sapiens (Human)) | BDBM50376935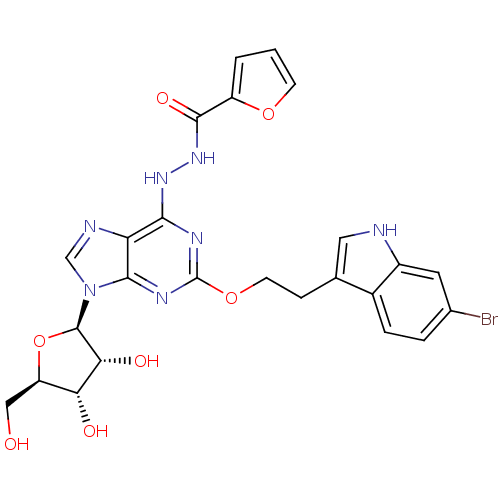 (CHEMBL429684) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 289 | n/a | n/a | n/a | n/a |
National Institute of Diabetes and Digestive and Kidney Diseases Curated by ChEMBL | Assay Description Agonist activity at human adenosine A2B receptor expressed in CHO cells assessed as stimulation of adenylate cyclase | J Med Chem 51: 2088-99 (2008) Article DOI: 10.1021/jm701442d BindingDB Entry DOI: 10.7270/Q2R49RNV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A2b (Homo sapiens (Human)) | BDBM50376932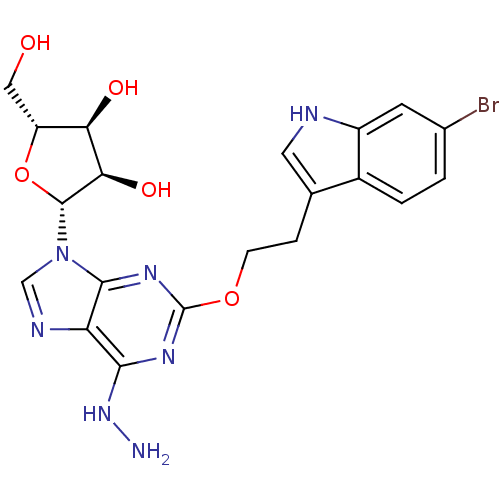 (CHEMBL410104) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 174 | n/a | n/a | n/a | n/a |
National Institute of Diabetes and Digestive and Kidney Diseases Curated by ChEMBL | Assay Description Agonist activity at human adenosine A2B receptor expressed in CHO cells assessed as stimulation of adenylate cyclase | J Med Chem 51: 2088-99 (2008) Article DOI: 10.1021/jm701442d BindingDB Entry DOI: 10.7270/Q2R49RNV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A2b (Homo sapiens (Human)) | BDBM50003261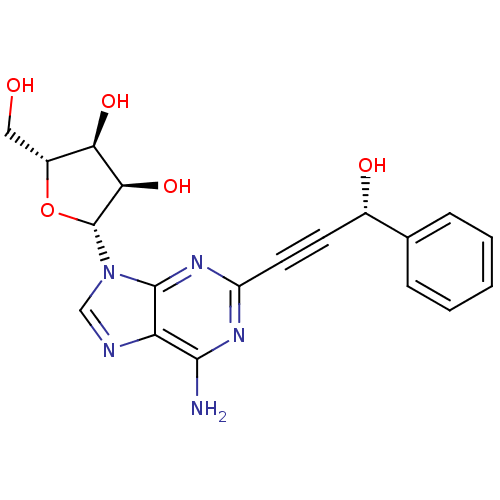 (CHEMBL408918) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | n/a | n/a | 1.61E+5 | n/a | n/a | n/a | n/a |
National Institute of Diabetes and Digestive and Kidney Diseases Curated by ChEMBL | Assay Description Agonist activity at human adenosine A2B receptor expressed in CHO cells assessed as stimulation of adenylate cyclase | J Med Chem 51: 2088-99 (2008) Article DOI: 10.1021/jm701442d BindingDB Entry DOI: 10.7270/Q2R49RNV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A2b (Homo sapiens (Human)) | BDBM50003259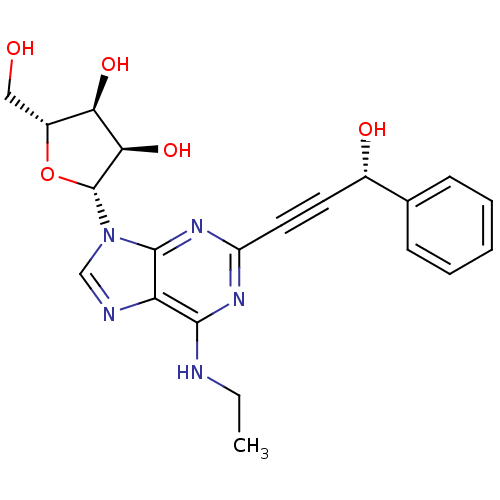 (CHEMBL410095) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 1.10E+5 | n/a | n/a | n/a | n/a |
National Institute of Diabetes and Digestive and Kidney Diseases Curated by ChEMBL | Assay Description Agonist activity at human adenosine A2B receptor expressed in CHO cells assessed as stimulation of adenylate cyclase | J Med Chem 51: 2088-99 (2008) Article DOI: 10.1021/jm701442d BindingDB Entry DOI: 10.7270/Q2R49RNV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A2b (Homo sapiens (Human)) | BDBM50003260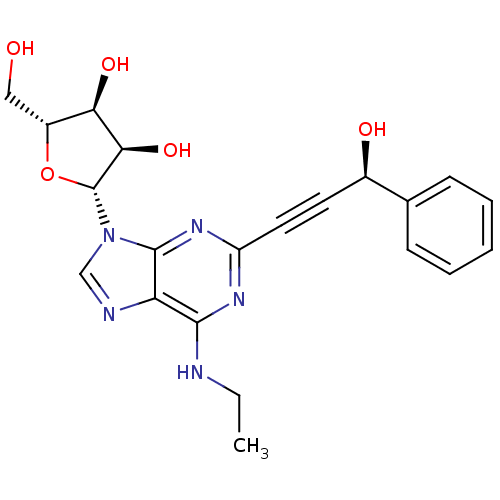 (CHEMBL258535) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | n/a | n/a | 1.37E+6 | n/a | n/a | n/a | n/a |
National Institute of Diabetes and Digestive and Kidney Diseases Curated by ChEMBL | Assay Description Agonist activity at human adenosine A2B receptor expressed in CHO cells assessed as stimulation of adenylate cyclase | J Med Chem 51: 2088-99 (2008) Article DOI: 10.1021/jm701442d BindingDB Entry DOI: 10.7270/Q2R49RNV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A2b (Homo sapiens (Human)) | BDBM50003258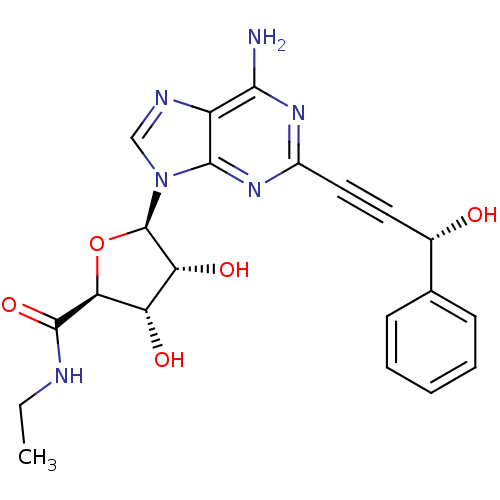 (CHEMBL258534) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 4.17E+5 | n/a | n/a | n/a | n/a |
National Institute of Diabetes and Digestive and Kidney Diseases Curated by ChEMBL | Assay Description Agonist activity at human adenosine A2B receptor expressed in CHO cells assessed as stimulation of adenylate cyclase | J Med Chem 51: 2088-99 (2008) Article DOI: 10.1021/jm701442d BindingDB Entry DOI: 10.7270/Q2R49RNV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A2b (Homo sapiens (Human)) | BDBM50003256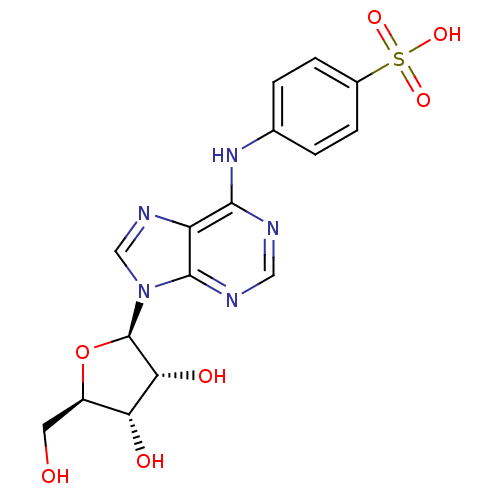 (CHEMBL411732) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 1.04E+4 | n/a | n/a | n/a | n/a |
National Institute of Diabetes and Digestive and Kidney Diseases Curated by ChEMBL | Assay Description Agonist activity at human adenosine A2B receptor expressed in CHO cells assessed as stimulation of adenylate cyclase | J Med Chem 51: 2088-99 (2008) Article DOI: 10.1021/jm701442d BindingDB Entry DOI: 10.7270/Q2R49RNV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A2b (Homo sapiens (Human)) | BDBM50208809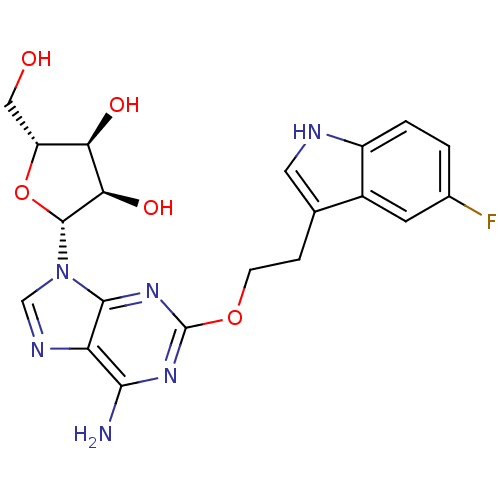 ((2R,3R,4S,5R)-2-(6-amino-2-(2-(5-fluoro-1H-indol-3...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 1.30E+6 | n/a | n/a | n/a | n/a |
National Institute of Diabetes and Digestive and Kidney Diseases Curated by ChEMBL | Assay Description Agonist activity at human adenosine A2B receptor expressed in CHO cells assessed as stimulation of adenylate cyclase | J Med Chem 51: 2088-99 (2008) Article DOI: 10.1021/jm701442d BindingDB Entry DOI: 10.7270/Q2R49RNV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A2b (Homo sapiens (Human)) | BDBM50003267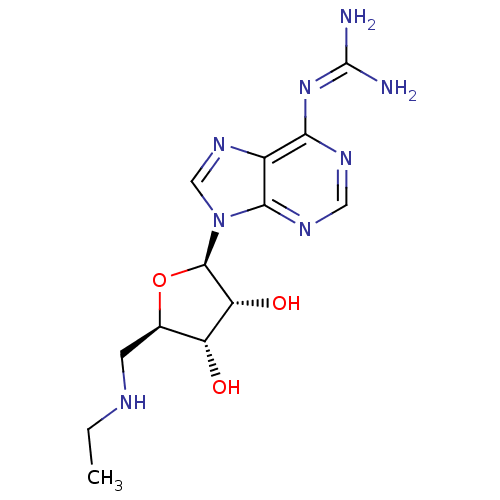 (6-guanidino- NECA | CHEMBL405144) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 1.84E+7 | n/a | n/a | n/a | n/a |
National Institute of Diabetes and Digestive and Kidney Diseases Curated by ChEMBL | Assay Description Agonist activity at human adenosine A2B receptor expressed in CHO cells assessed as stimulation of adenylate cyclase | J Med Chem 51: 2088-99 (2008) Article DOI: 10.1021/jm701442d BindingDB Entry DOI: 10.7270/Q2R49RNV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A2b (Homo sapiens (Human)) | BDBM50364044 (CHEMBL260203) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 6.95E+5 | n/a | n/a | n/a | n/a |
National Institute of Diabetes and Digestive and Kidney Diseases Curated by ChEMBL | Assay Description Agonist activity at human adenosine A2B receptor expressed in CHO cells assessed as stimulation of adenylate cyclase | J Med Chem 51: 2088-99 (2008) Article DOI: 10.1021/jm701442d BindingDB Entry DOI: 10.7270/Q2R49RNV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A2b (Homo sapiens (Human)) | BDBM42467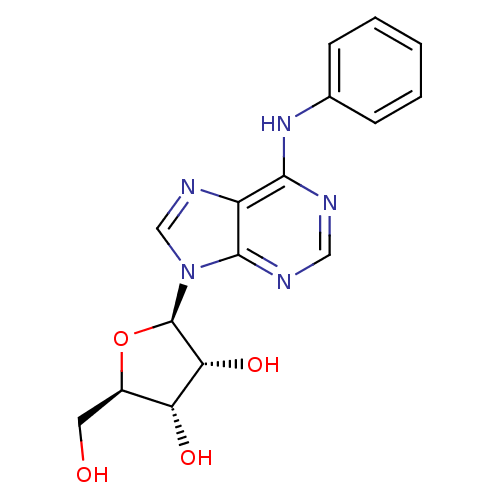 ((2R,3R,4S,5R)-2-(6-anilino-9-purinyl)-5-(hydroxyme...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 4.92E+3 | n/a | n/a | n/a | n/a |
National Institute of Diabetes and Digestive and Kidney Diseases Curated by ChEMBL | Assay Description Agonist activity at human adenosine A2B receptor expressed in CHO cells assessed as stimulation of adenylate cyclase | J Med Chem 51: 2088-99 (2008) Article DOI: 10.1021/jm701442d BindingDB Entry DOI: 10.7270/Q2R49RNV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A2b (Homo sapiens (Human)) | BDBM50208788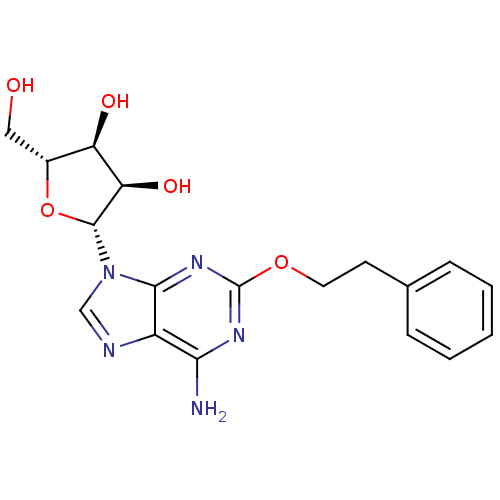 ((2R,3R,4S,5R)-2-(6-amino-2-phenethoxy-9H-purin-9-y...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | n/a | n/a | 2.86E+5 | n/a | n/a | n/a | n/a |
National Institute of Diabetes and Digestive and Kidney Diseases Curated by ChEMBL | Assay Description Agonist activity at human adenosine A2B receptor expressed in CHO cells assessed as stimulation of adenylate cyclase | J Med Chem 51: 2088-99 (2008) Article DOI: 10.1021/jm701442d BindingDB Entry DOI: 10.7270/Q2R49RNV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A2b (Homo sapiens (Human)) | BDBM50003281 (CHEMBL438418) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | n/a | n/a | 1.12E+5 | n/a | n/a | n/a | n/a |
National Institute of Diabetes and Digestive and Kidney Diseases Curated by ChEMBL | Assay Description Agonist activity at human adenosine A2B receptor expressed in CHO cells assessed as stimulation of adenylate cyclase | J Med Chem 51: 2088-99 (2008) Article DOI: 10.1021/jm701442d BindingDB Entry DOI: 10.7270/Q2R49RNV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A2b (Homo sapiens (Human)) | BDBM50208786 ((2R,3R,4S,5R)-2-(6-amino-2-(2-(6-chloro-1H-indol-3...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 4.62E+6 | n/a | n/a | n/a | n/a |
National Institute of Diabetes and Digestive and Kidney Diseases Curated by ChEMBL | Assay Description Agonist activity at human adenosine A2B receptor expressed in CHO cells assessed as stimulation of adenylate cyclase | J Med Chem 51: 2088-99 (2008) Article DOI: 10.1021/jm701442d BindingDB Entry DOI: 10.7270/Q2R49RNV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A2b (Homo sapiens (Human)) | BDBM50119168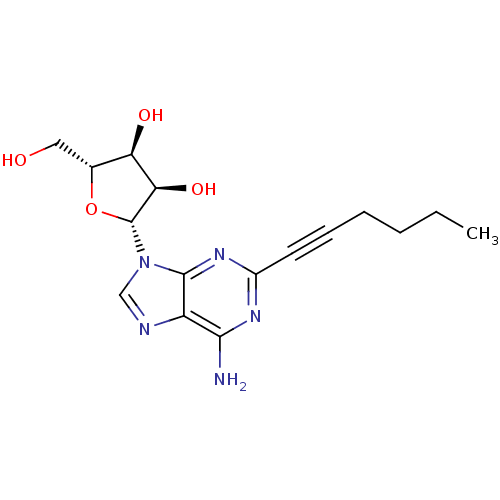 ((2R,3R,4S,5R)-2-(6-Amino-2-hex-1-ynyl-purin-9-yl)-...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 1.00E+4 | n/a | n/a | n/a | n/a |
National Institute of Diabetes and Digestive and Kidney Diseases Curated by ChEMBL | Assay Description Agonist activity at human adenosine A2B receptor expressed in CHO cells assessed as stimulation of adenylate cyclase | J Med Chem 51: 2088-99 (2008) Article DOI: 10.1021/jm701442d BindingDB Entry DOI: 10.7270/Q2R49RNV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A2b (Homo sapiens (Human)) | BDBM50208800 ((2R,3R,4S,5R)-2-(6-amino-2-(2-(thiophen-2-yl)ethox...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | n/a | n/a | 5.62E+5 | n/a | n/a | n/a | n/a |
National Institute of Diabetes and Digestive and Kidney Diseases Curated by ChEMBL | Assay Description Agonist activity at human adenosine A2B receptor expressed in CHO cells assessed as stimulation of adenylate cyclase | J Med Chem 51: 2088-99 (2008) Article DOI: 10.1021/jm701442d BindingDB Entry DOI: 10.7270/Q2R49RNV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A2b (Homo sapiens (Human)) | BDBM50205588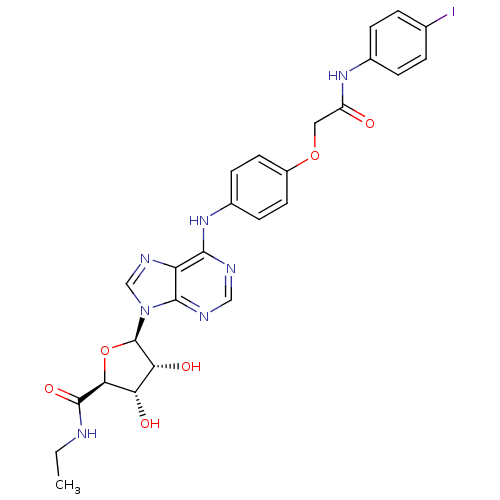 ((2S,3S,4R,5R)-N-ethyl-3,4-dihydroxy-5-(6-(4-(2-(4-...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 3.31E+7 | n/a | n/a | n/a | n/a |
National Institute of Diabetes and Digestive and Kidney Diseases Curated by ChEMBL | Assay Description Agonist activity at human adenosine A2B receptor expressed in CHO cells assessed as stimulation of adenylate cyclase | J Med Chem 51: 2088-99 (2008) Article DOI: 10.1021/jm701442d BindingDB Entry DOI: 10.7270/Q2R49RNV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A2b (Homo sapiens (Human)) | BDBM50205580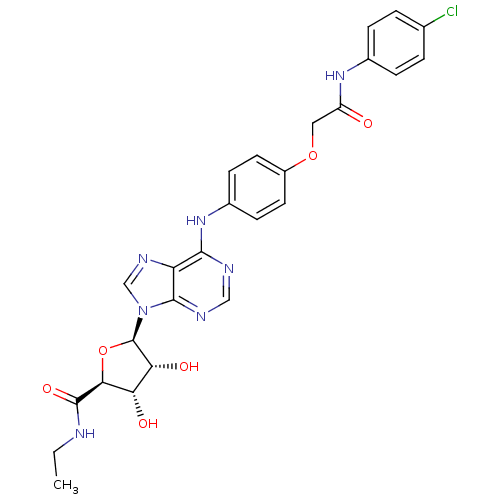 ((2S,3S,4R,5R)-5-(6-(4-(2-(4-chlorophenylamino)-2-o...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 8.13E+7 | n/a | n/a | n/a | n/a |
National Institute of Diabetes and Digestive and Kidney Diseases Curated by ChEMBL | Assay Description Agonist activity at human adenosine A2B receptor expressed in CHO cells assessed as stimulation of adenylate cyclase | J Med Chem 51: 2088-99 (2008) Article DOI: 10.1021/jm701442d BindingDB Entry DOI: 10.7270/Q2R49RNV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A2b (Homo sapiens (Human)) | BDBM50009552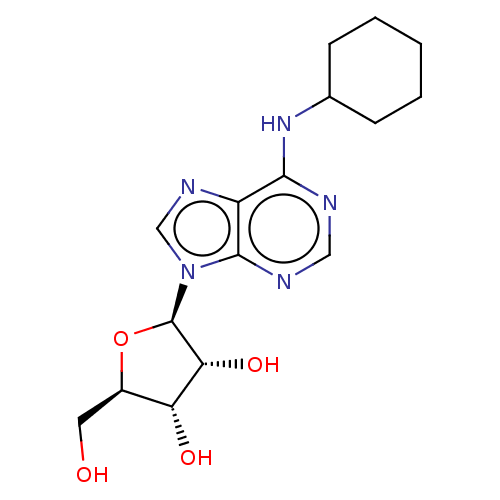 (2-[6-Amino-2-(2-morpholin-4-yl-ethylamino)-purin-9...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 5.26E+4 | n/a | n/a | n/a | n/a |
National Institute of Diabetes and Digestive and Kidney Diseases Curated by ChEMBL | Assay Description Agonist activity at human adenosine A2B receptor expressed in CHO cells assessed as stimulation of adenylate cyclase | J Med Chem 51: 2088-99 (2008) Article DOI: 10.1021/jm701442d BindingDB Entry DOI: 10.7270/Q2R49RNV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A2b (Homo sapiens (Human)) | BDBM50202570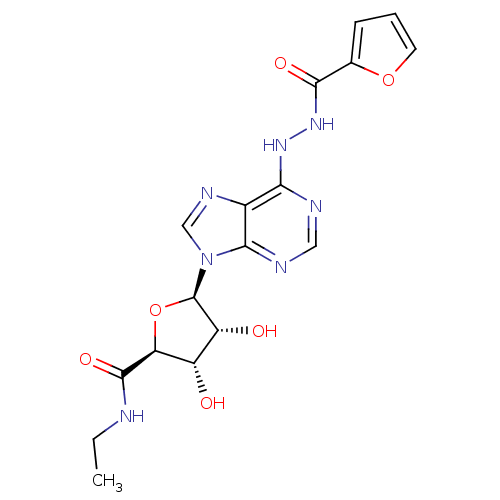 (1-deoxy-1-{6-[N'-(furan-2-carbonyl)-hydrazino]-9H-...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 1.22E+7 | n/a | n/a | n/a | n/a |
National Institute of Diabetes and Digestive and Kidney Diseases Curated by ChEMBL | Assay Description Agonist activity at human adenosine A2B receptor expressed in CHO cells assessed as stimulation of adenylate cyclase | J Med Chem 51: 2088-99 (2008) Article DOI: 10.1021/jm701442d BindingDB Entry DOI: 10.7270/Q2R49RNV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A2b (Homo sapiens (Human)) | BDBM50205587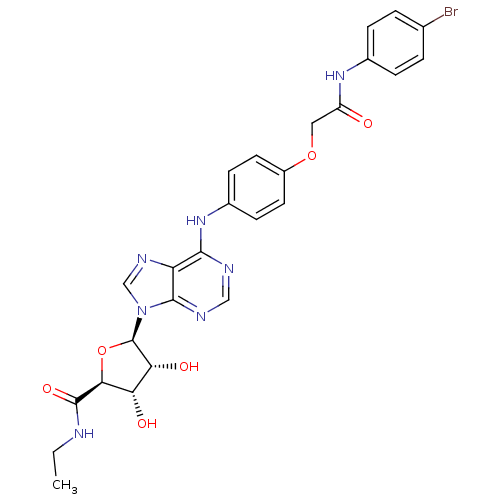 ((2S,3S,4R,5R)-5-(6-(4-(2-(4-bromophenylamino)-2-ox...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 9.53E+7 | n/a | n/a | n/a | n/a |
National Institute of Diabetes and Digestive and Kidney Diseases Curated by ChEMBL | Assay Description Agonist activity at human adenosine A2B receptor expressed in CHO cells assessed as stimulation of adenylate cyclase | J Med Chem 51: 2088-99 (2008) Article DOI: 10.1021/jm701442d BindingDB Entry DOI: 10.7270/Q2R49RNV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A2b (Homo sapiens (Human)) | BDBM50205591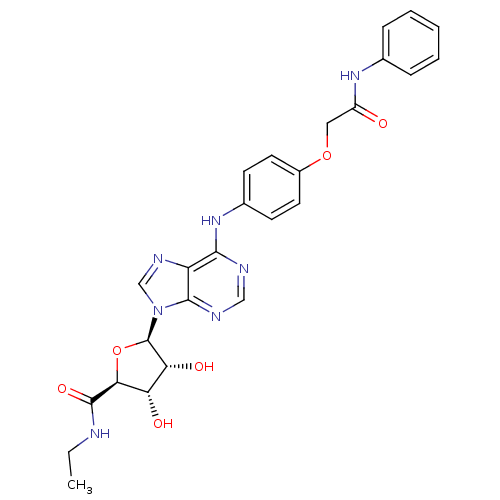 ((2S,3S,4R,5R)-N-ethyl-3,4-dihydroxy-5-(6-(4-(2-oxo...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 1.37E+8 | n/a | n/a | n/a | n/a |
National Institute of Diabetes and Digestive and Kidney Diseases Curated by ChEMBL | Assay Description Agonist activity at human adenosine A2B receptor expressed in CHO cells assessed as stimulation of adenylate cyclase | J Med Chem 51: 2088-99 (2008) Article DOI: 10.1021/jm701442d BindingDB Entry DOI: 10.7270/Q2R49RNV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A2b (Homo sapiens (Human)) | BDBM50205585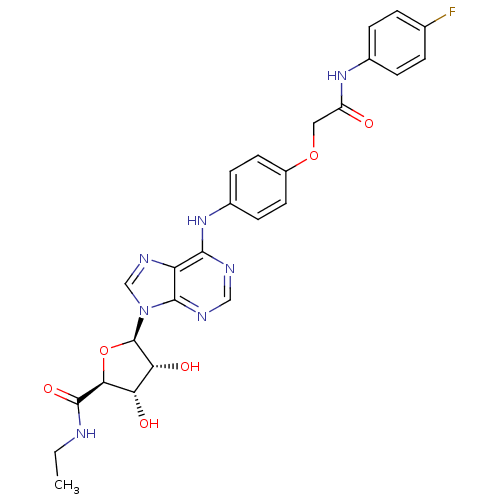 ((2S,3S,4R,5R)-N-ethyl-5-(6-(4-(2-(4-fluorophenylam...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 6.58E+7 | n/a | n/a | n/a | n/a |
National Institute of Diabetes and Digestive and Kidney Diseases Curated by ChEMBL | Assay Description Agonist activity at human adenosine A2B receptor expressed in CHO cells assessed as stimulation of adenylate cyclase | J Med Chem 51: 2088-99 (2008) Article DOI: 10.1021/jm701442d BindingDB Entry DOI: 10.7270/Q2R49RNV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A2b (Homo sapiens (Human)) | BDBM21220 ((2S,3S,4R,5R)-5-(6-amino-9H-purin-9-yl)-N-ethyl-3,...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE MMDB PC cid PC sid PDB UniChem Similars | PDB Article PubMed | n/a | n/a | n/a | n/a | 7.14E+6 | n/a | n/a | n/a | n/a |
National Institute of Diabetes and Digestive and Kidney Diseases Curated by ChEMBL | Assay Description Agonist activity at human adenosine A2B receptor expressed in CHO cells assessed as stimulation of adenylate cyclase | J Med Chem 51: 2088-99 (2008) Article DOI: 10.1021/jm701442d BindingDB Entry DOI: 10.7270/Q2R49RNV | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Adenosine receptor A2b (Homo sapiens (Human)) | BDBM50003274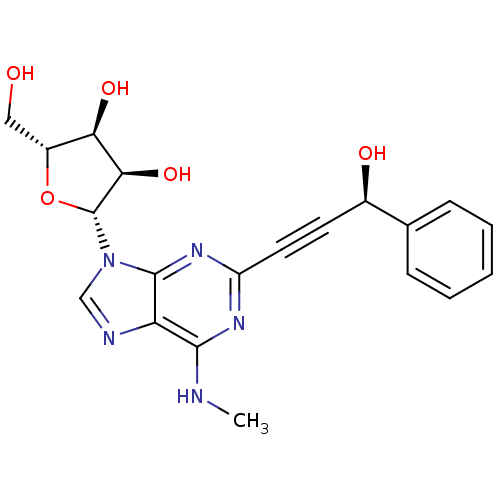 (CHEMBL261453) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 3.85E+5 | n/a | n/a | n/a | n/a |
National Institute of Diabetes and Digestive and Kidney Diseases Curated by ChEMBL | Assay Description Agonist activity at human adenosine A2B receptor expressed in CHO cells assessed as stimulation of adenylate cyclase | J Med Chem 51: 2088-99 (2008) Article DOI: 10.1021/jm701442d BindingDB Entry DOI: 10.7270/Q2R49RNV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A2b (Homo sapiens (Human)) | BDBM50202575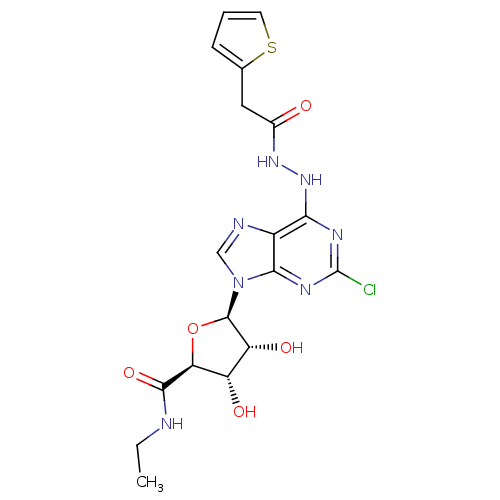 (1-deoxy-1-{2-chloro-6-[N'-(2-thiophen-2-yl-acetyl)...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 1.66E+6 | n/a | n/a | n/a | n/a |
National Institute of Diabetes and Digestive and Kidney Diseases Curated by ChEMBL | Assay Description Agonist activity at human adenosine A2B receptor expressed in CHO cells assessed as stimulation of adenylate cyclase | J Med Chem 51: 2088-99 (2008) Article DOI: 10.1021/jm701442d BindingDB Entry DOI: 10.7270/Q2R49RNV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A2b (Homo sapiens (Human)) | BDBM50003280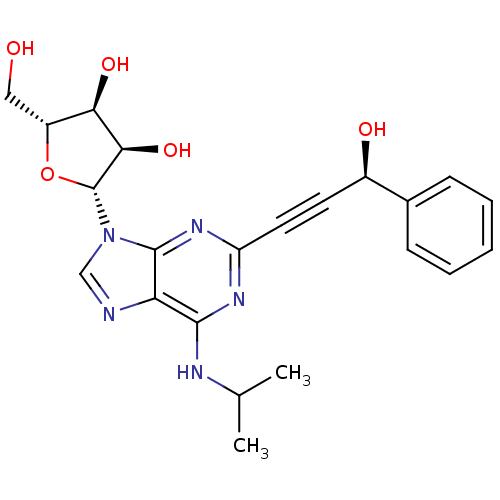 (CHEMBL259938) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 1.04E+6 | n/a | n/a | n/a | n/a |
National Institute of Diabetes and Digestive and Kidney Diseases Curated by ChEMBL | Assay Description Agonist activity at human adenosine A2B receptor expressed in CHO cells assessed as stimulation of adenylate cyclase | J Med Chem 51: 2088-99 (2008) Article DOI: 10.1021/jm701442d BindingDB Entry DOI: 10.7270/Q2R49RNV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A2b (Homo sapiens (Human)) | BDBM50202565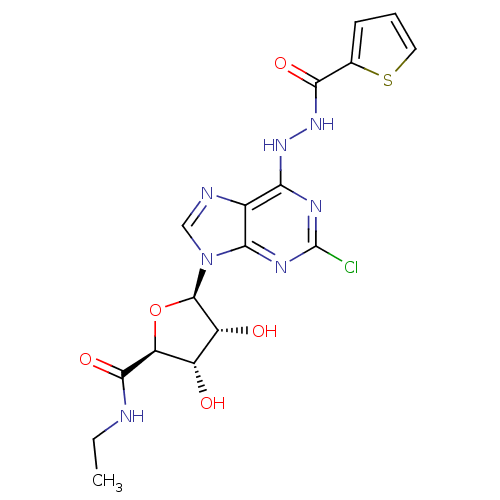 (1-deoxy-1-{2-chloro-6-[N'-(thiophene-2-carbonyl)-h...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 5.00E+6 | n/a | n/a | n/a | n/a |
National Institute of Diabetes and Digestive and Kidney Diseases Curated by ChEMBL | Assay Description Agonist activity at human adenosine A2B receptor expressed in CHO cells assessed as stimulation of adenylate cyclase | J Med Chem 51: 2088-99 (2008) Article DOI: 10.1021/jm701442d BindingDB Entry DOI: 10.7270/Q2R49RNV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A2b (Homo sapiens (Human)) | BDBM50202568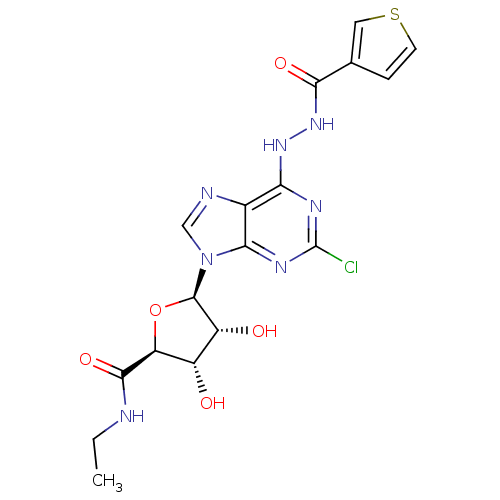 (1-deoxy-1-{2-chloro-6-[N'-(thiophene-3-carbonyl)-h...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 2.22E+6 | n/a | n/a | n/a | n/a |
National Institute of Diabetes and Digestive and Kidney Diseases Curated by ChEMBL | Assay Description Agonist activity at human adenosine A2B receptor expressed in CHO cells assessed as stimulation of adenylate cyclase | J Med Chem 51: 2088-99 (2008) Article DOI: 10.1021/jm701442d BindingDB Entry DOI: 10.7270/Q2R49RNV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A2b (Homo sapiens (Human)) | BDBM50003270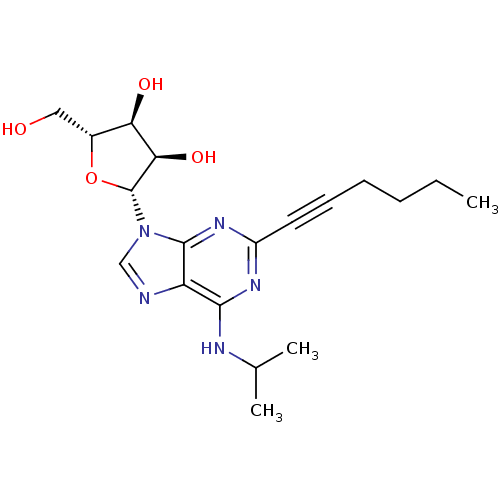 (CHEMBL261623) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | n/a | n/a | 1.00E+4 | n/a | n/a | n/a | n/a |
National Institute of Diabetes and Digestive and Kidney Diseases Curated by ChEMBL | Assay Description Agonist activity at human adenosine A2B receptor expressed in CHO cells assessed as stimulation of adenylate cyclase | J Med Chem 51: 2088-99 (2008) Article DOI: 10.1021/jm701442d BindingDB Entry DOI: 10.7270/Q2R49RNV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A2b (Homo sapiens (Human)) | BDBM50205586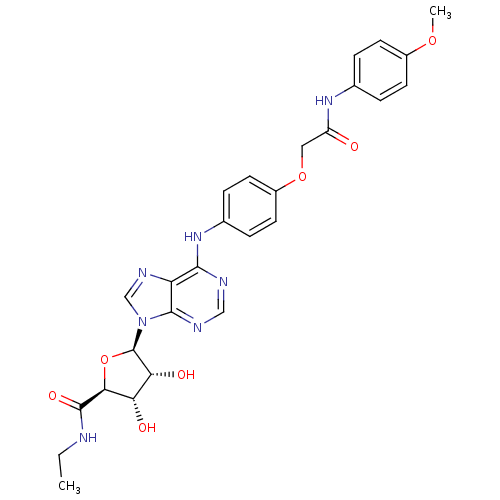 ((2S,3S,4R,5R)-N-ethyl-3,4-dihydroxy-5-(6-(4-(2-(4-...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 3.08E+7 | n/a | n/a | n/a | n/a |
National Institute of Diabetes and Digestive and Kidney Diseases Curated by ChEMBL | Assay Description Agonist activity at human adenosine A2B receptor expressed in CHO cells assessed as stimulation of adenylate cyclase | J Med Chem 51: 2088-99 (2008) Article DOI: 10.1021/jm701442d BindingDB Entry DOI: 10.7270/Q2R49RNV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A2b (Homo sapiens (Human)) | BDBM50389797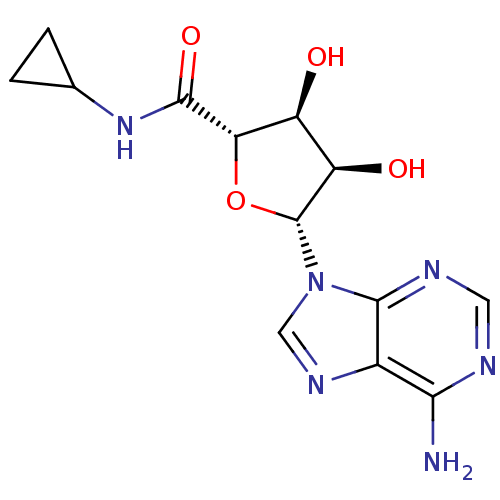 (CHEMBL261482) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 3.23E+5 | n/a | n/a | n/a | n/a |
National Institute of Diabetes and Digestive and Kidney Diseases Curated by ChEMBL | Assay Description Agonist activity at human adenosine A2B receptor expressed in CHO cells assessed as stimulation of adenylate cyclase | J Med Chem 51: 2088-99 (2008) Article DOI: 10.1021/jm701442d BindingDB Entry DOI: 10.7270/Q2R49RNV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A2b (Homo sapiens (Human)) | BDBM50003268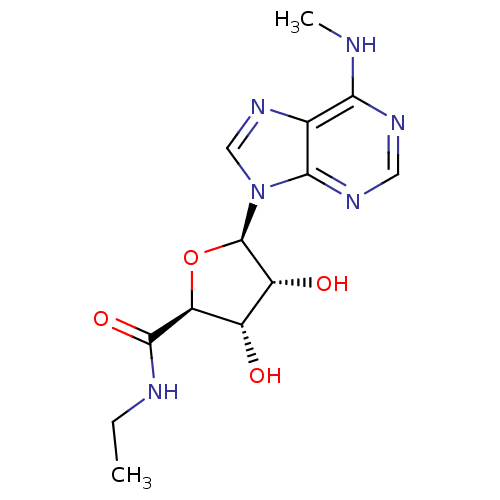 (CHEMBL407502) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 4.17E+4 | n/a | n/a | n/a | n/a |
National Institute of Diabetes and Digestive and Kidney Diseases Curated by ChEMBL | Assay Description Agonist activity at human adenosine A2B receptor expressed in CHO cells assessed as stimulation of adenylate cyclase | J Med Chem 51: 2088-99 (2008) Article DOI: 10.1021/jm701442d BindingDB Entry DOI: 10.7270/Q2R49RNV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A2b (Homo sapiens (Human)) | BDBM50003273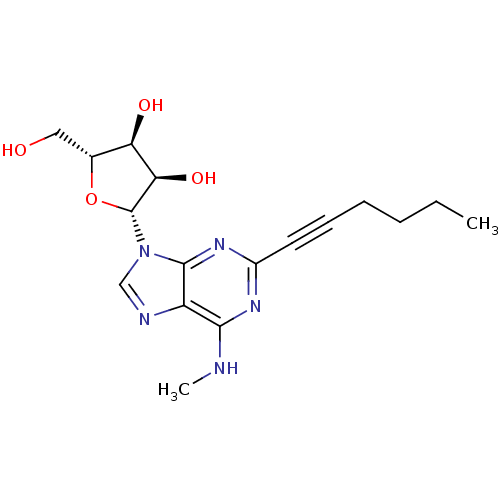 (CHEMBL260563) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 1.00E+4 | n/a | n/a | n/a | n/a |
National Institute of Diabetes and Digestive and Kidney Diseases Curated by ChEMBL | Assay Description Agonist activity at human adenosine A2B receptor expressed in CHO cells assessed as stimulation of adenylate cyclase | J Med Chem 51: 2088-99 (2008) Article DOI: 10.1021/jm701442d BindingDB Entry DOI: 10.7270/Q2R49RNV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A2b (Homo sapiens (Human)) | BDBM50003272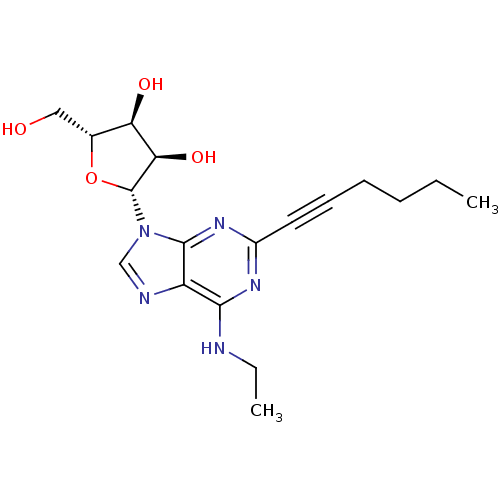 (CHEMBL437842) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 1.00E+4 | n/a | n/a | n/a | n/a |
National Institute of Diabetes and Digestive and Kidney Diseases Curated by ChEMBL | Assay Description Agonist activity at human adenosine A2B receptor expressed in CHO cells assessed as stimulation of adenylate cyclase | J Med Chem 51: 2088-99 (2008) Article DOI: 10.1021/jm701442d BindingDB Entry DOI: 10.7270/Q2R49RNV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A2b (Homo sapiens (Human)) | BDBM50205589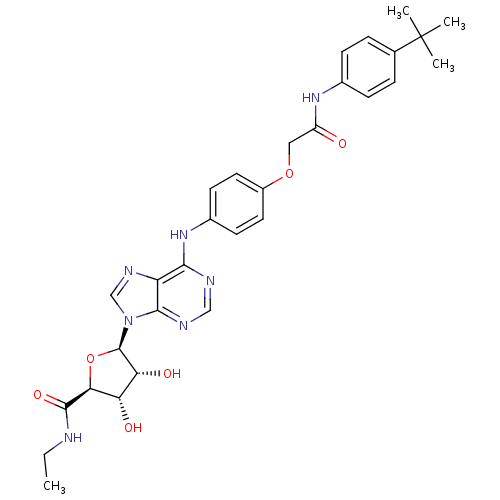 ((2S,3S,4R,5R)-5-(6-(4-(2-(4-tert-butylphenylamino)...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 6.10E+7 | n/a | n/a | n/a | n/a |
National Institute of Diabetes and Digestive and Kidney Diseases Curated by ChEMBL | Assay Description Agonist activity at human adenosine A2B receptor expressed in CHO cells assessed as stimulation of adenylate cyclase | J Med Chem 51: 2088-99 (2008) Article DOI: 10.1021/jm701442d BindingDB Entry DOI: 10.7270/Q2R49RNV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A2b (Homo sapiens (Human)) | BDBM50205584 ((2S,3S,4R,5R)-5-(6-(4-(2-(benzo[d][1,3]dioxol-5-yl...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 2.82E+7 | n/a | n/a | n/a | n/a |
National Institute of Diabetes and Digestive and Kidney Diseases Curated by ChEMBL | Assay Description Agonist activity at human adenosine A2B receptor expressed in CHO cells assessed as stimulation of adenylate cyclase | J Med Chem 51: 2088-99 (2008) Article DOI: 10.1021/jm701442d BindingDB Entry DOI: 10.7270/Q2R49RNV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A2b (Homo sapiens (Human)) | BDBM50205590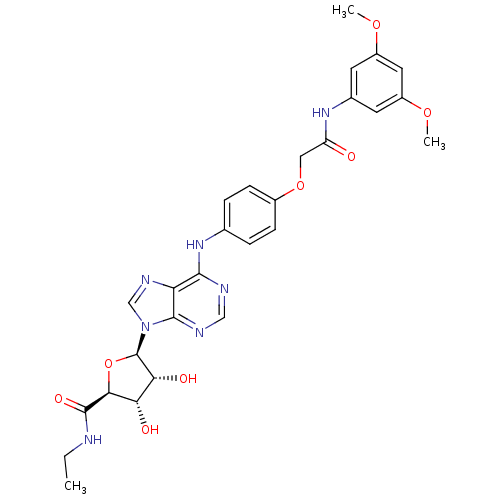 ((2S,3S,4R,5R)-5-(6-(4-(2-(3,5-dimethoxyphenylamino...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 1.87E+7 | n/a | n/a | n/a | n/a |
National Institute of Diabetes and Digestive and Kidney Diseases Curated by ChEMBL | Assay Description Agonist activity at human adenosine A2B receptor expressed in CHO cells assessed as stimulation of adenylate cyclase | J Med Chem 51: 2088-99 (2008) Article DOI: 10.1021/jm701442d BindingDB Entry DOI: 10.7270/Q2R49RNV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A2b (Homo sapiens (Human)) | BDBM50205593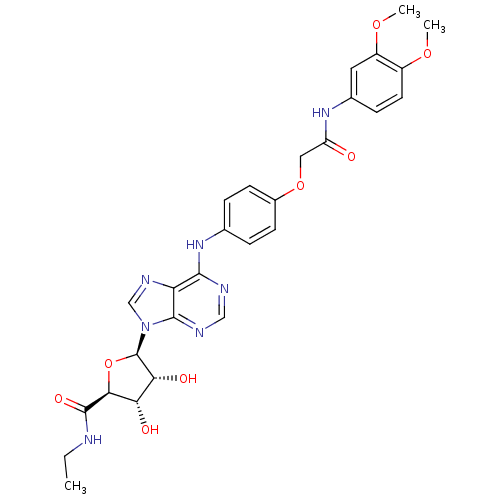 ((2S,3S,4R,5R)-5-(6-(4-(2-(3,4-dimethoxyphenylamino...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 2.20E+7 | n/a | n/a | n/a | n/a |
National Institute of Diabetes and Digestive and Kidney Diseases Curated by ChEMBL | Assay Description Agonist activity at human adenosine A2B receptor expressed in CHO cells assessed as stimulation of adenylate cyclase | J Med Chem 51: 2088-99 (2008) Article DOI: 10.1021/jm701442d BindingDB Entry DOI: 10.7270/Q2R49RNV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A2b (Homo sapiens (Human)) | BDBM50205576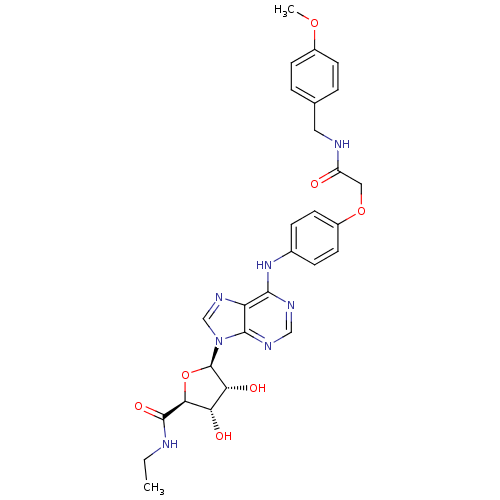 ((2S,3S,4R,5R)-5-(6-(4-(2-(4-methoxybenzylamino)-2-...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 4.17E+6 | n/a | n/a | n/a | n/a |
National Institute of Diabetes and Digestive and Kidney Diseases Curated by ChEMBL | Assay Description Agonist activity at human adenosine A2B receptor expressed in CHO cells assessed as stimulation of adenylate cyclase | J Med Chem 51: 2088-99 (2008) Article DOI: 10.1021/jm701442d BindingDB Entry DOI: 10.7270/Q2R49RNV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A2b (Homo sapiens (Human)) | BDBM50003277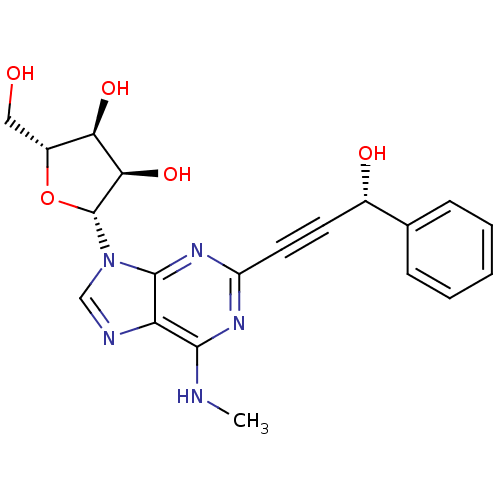 (CHEMBL410115) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 1.00E+4 | n/a | n/a | n/a | n/a |
National Institute of Diabetes and Digestive and Kidney Diseases Curated by ChEMBL | Assay Description Agonist activity at human adenosine A2B receptor expressed in CHO cells assessed as stimulation of adenylate cyclase | J Med Chem 51: 2088-99 (2008) Article DOI: 10.1021/jm701442d BindingDB Entry DOI: 10.7270/Q2R49RNV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A2b (Homo sapiens (Human)) | BDBM50003276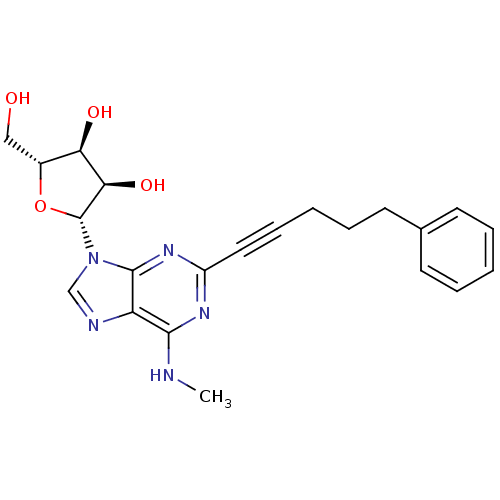 (CHEMBL260041) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | n/a | n/a | 1.00E+4 | n/a | n/a | n/a | n/a |
National Institute of Diabetes and Digestive and Kidney Diseases Curated by ChEMBL | Assay Description Agonist activity at human adenosine A2B receptor expressed in CHO cells assessed as stimulation of adenylate cyclase | J Med Chem 51: 2088-99 (2008) Article DOI: 10.1021/jm701442d BindingDB Entry DOI: 10.7270/Q2R49RNV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A2b (Homo sapiens (Human)) | BDBM50205583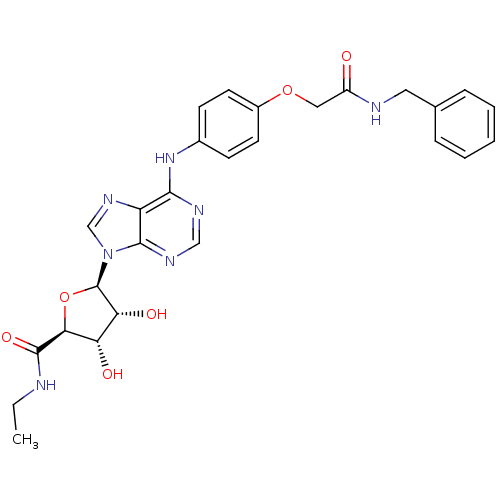 ((2S,3S,4R,5R)-5-(6-(4-(2-(benzylamino)-2-oxoethoxy...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 6.67E+6 | n/a | n/a | n/a | n/a |
National Institute of Diabetes and Digestive and Kidney Diseases Curated by ChEMBL | Assay Description Agonist activity at human adenosine A2B receptor expressed in CHO cells assessed as stimulation of adenylate cyclase | J Med Chem 51: 2088-99 (2008) Article DOI: 10.1021/jm701442d BindingDB Entry DOI: 10.7270/Q2R49RNV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A2b (Homo sapiens (Human)) | BDBM50205579 ((2S,3S,4R,5R)-N-ethyl-3,4-dihydroxy-5-(6-(4-(2-oxo...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 3.10E+7 | n/a | n/a | n/a | n/a |
National Institute of Diabetes and Digestive and Kidney Diseases Curated by ChEMBL | Assay Description Agonist activity at human adenosine A2B receptor expressed in CHO cells assessed as stimulation of adenylate cyclase | J Med Chem 51: 2088-99 (2008) Article DOI: 10.1021/jm701442d BindingDB Entry DOI: 10.7270/Q2R49RNV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 80 total ) | Next | Last >> |