

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Chymase (Homo sapiens (Human)) | BDBM134252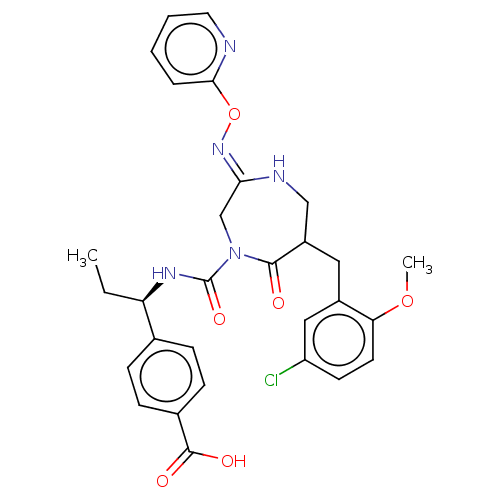 (US8846660, 3) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 30 | n/a | n/a | n/a | n/a | 7.5 | 25 |
Daiichi Sankyo Company, Ltd. US Patent | Assay Description The inhibitory activity of the compounds of the present invention for recombinant human chymase was measured by the method of Pasztor et al. (Pasztor... | US Patent US8846660 (2014) BindingDB Entry DOI: 10.7270/Q22V2DTB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Chymase (Homo sapiens (Human)) | BDBM134281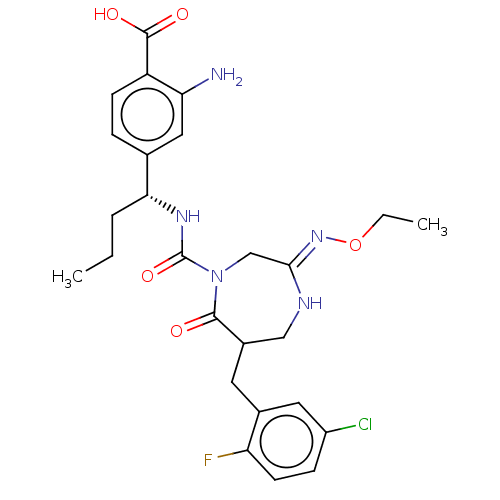 (US8846660, 113) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 40 | n/a | n/a | n/a | n/a | 7.5 | 25 |
Daiichi Sankyo Company, Ltd. US Patent | Assay Description The inhibitory activity of the compounds of the present invention for recombinant human chymase was measured by the method of Pasztor et al. (Pasztor... | US Patent US8846660 (2014) BindingDB Entry DOI: 10.7270/Q22V2DTB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Chymase (Homo sapiens (Human)) | BDBM134254 (US8846660, 14) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 30 | n/a | n/a | n/a | n/a | 7.5 | 25 |
Daiichi Sankyo Company, Ltd. US Patent | Assay Description The inhibitory activity of the compounds of the present invention for recombinant human chymase was measured by the method of Pasztor et al. (Pasztor... | US Patent US8846660 (2014) BindingDB Entry DOI: 10.7270/Q22V2DTB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Chymase (Homo sapiens (Human)) | BDBM134255 (US8846660, 15) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 30 | n/a | n/a | n/a | n/a | 7.5 | 25 |
Daiichi Sankyo Company, Ltd. US Patent | Assay Description The inhibitory activity of the compounds of the present invention for recombinant human chymase was measured by the method of Pasztor et al. (Pasztor... | US Patent US8846660 (2014) BindingDB Entry DOI: 10.7270/Q22V2DTB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Chymase (Homo sapiens (Human)) | BDBM134256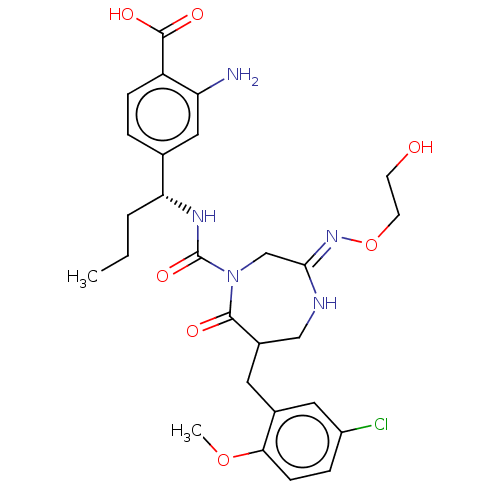 (US8846660, 18) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 20 | n/a | n/a | n/a | n/a | 7.5 | 25 |
Daiichi Sankyo Company, Ltd. US Patent | Assay Description The inhibitory activity of the compounds of the present invention for recombinant human chymase was measured by the method of Pasztor et al. (Pasztor... | US Patent US8846660 (2014) BindingDB Entry DOI: 10.7270/Q22V2DTB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Chymase (Homo sapiens (Human)) | BDBM134257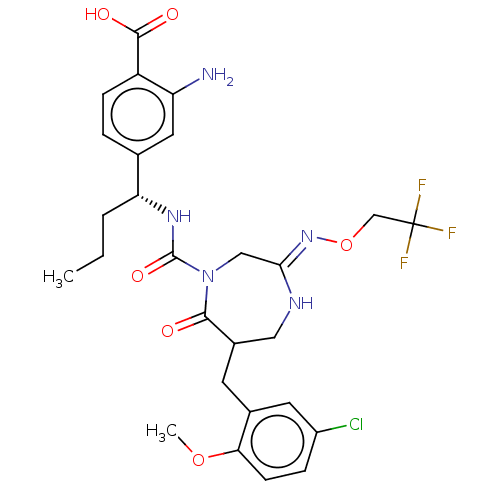 (US8846660, 20) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 30 | n/a | n/a | n/a | n/a | 7.5 | 25 |
Daiichi Sankyo Company, Ltd. US Patent | Assay Description The inhibitory activity of the compounds of the present invention for recombinant human chymase was measured by the method of Pasztor et al. (Pasztor... | US Patent US8846660 (2014) BindingDB Entry DOI: 10.7270/Q22V2DTB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Chymase (Homo sapiens (Human)) | BDBM134258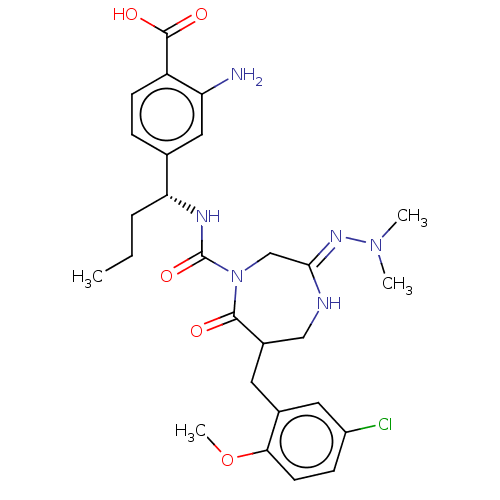 (US8846660, 22) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 40 | n/a | n/a | n/a | n/a | 7.5 | 25 |
Daiichi Sankyo Company, Ltd. US Patent | Assay Description The inhibitory activity of the compounds of the present invention for recombinant human chymase was measured by the method of Pasztor et al. (Pasztor... | US Patent US8846660 (2014) BindingDB Entry DOI: 10.7270/Q22V2DTB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Chymase (Homo sapiens (Human)) | BDBM134259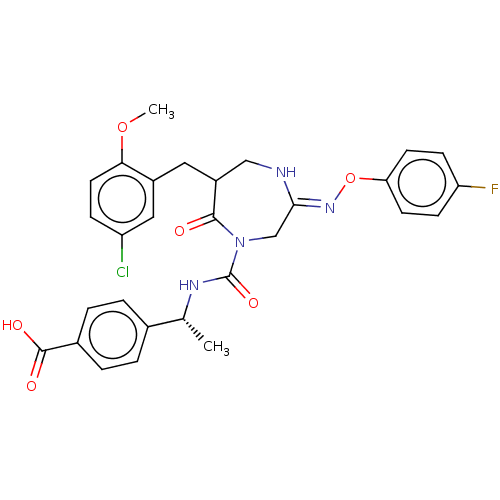 (US8846660, 26) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 50 | n/a | n/a | n/a | n/a | 7.5 | 25 |
Daiichi Sankyo Company, Ltd. US Patent | Assay Description The inhibitory activity of the compounds of the present invention for recombinant human chymase was measured by the method of Pasztor et al. (Pasztor... | US Patent US8846660 (2014) BindingDB Entry DOI: 10.7270/Q22V2DTB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Chymase (Homo sapiens (Human)) | BDBM134260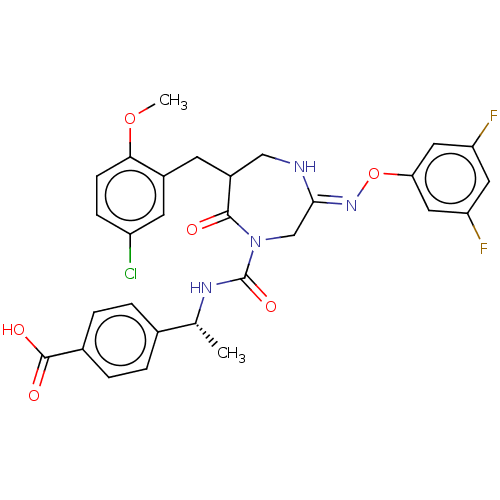 (US8846660, 27) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 20 | n/a | n/a | n/a | n/a | 7.5 | 25 |
Daiichi Sankyo Company, Ltd. US Patent | Assay Description The inhibitory activity of the compounds of the present invention for recombinant human chymase was measured by the method of Pasztor et al. (Pasztor... | US Patent US8846660 (2014) BindingDB Entry DOI: 10.7270/Q22V2DTB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Chymase (Homo sapiens (Human)) | BDBM134261 (US8846660, 29) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 10 | n/a | n/a | n/a | n/a | 7.5 | 25 |
Daiichi Sankyo Company, Ltd. US Patent | Assay Description The inhibitory activity of the compounds of the present invention for recombinant human chymase was measured by the method of Pasztor et al. (Pasztor... | US Patent US8846660 (2014) BindingDB Entry DOI: 10.7270/Q22V2DTB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Chymase (Homo sapiens (Human)) | BDBM134262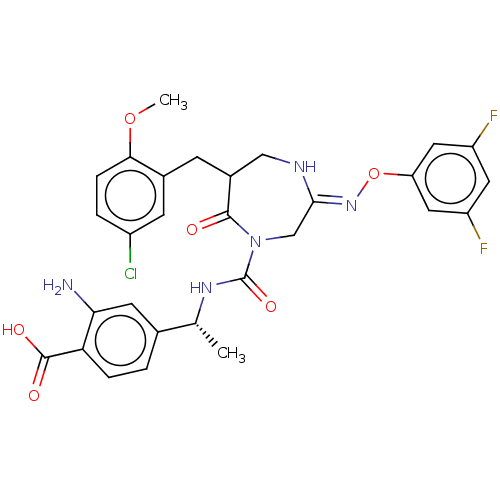 (US8846660, 30) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 4 | n/a | n/a | n/a | n/a | 7.5 | 25 |
Daiichi Sankyo Company, Ltd. US Patent | Assay Description The inhibitory activity of the compounds of the present invention for recombinant human chymase was measured by the method of Pasztor et al. (Pasztor... | US Patent US8846660 (2014) BindingDB Entry DOI: 10.7270/Q22V2DTB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Chymase (Homo sapiens (Human)) | BDBM134263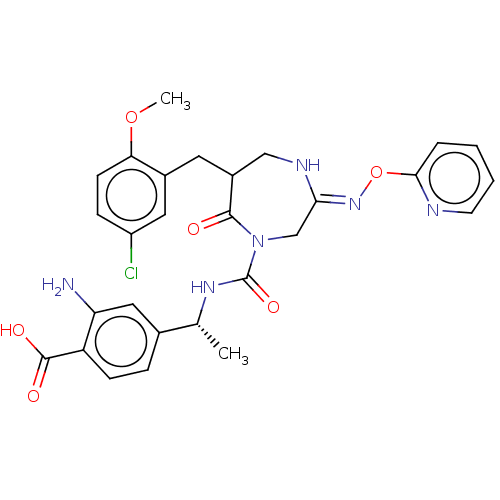 (US8846660, 31) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 10 | n/a | n/a | n/a | n/a | 7.5 | 25 |
Daiichi Sankyo Company, Ltd. US Patent | Assay Description The inhibitory activity of the compounds of the present invention for recombinant human chymase was measured by the method of Pasztor et al. (Pasztor... | US Patent US8846660 (2014) BindingDB Entry DOI: 10.7270/Q22V2DTB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Chymase (Homo sapiens (Human)) | BDBM134264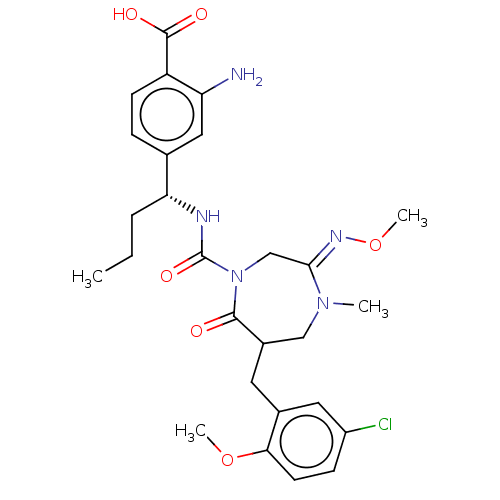 (US8846660, 34) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 30 | n/a | n/a | n/a | n/a | 7.5 | 25 |
Daiichi Sankyo Company, Ltd. US Patent | Assay Description The inhibitory activity of the compounds of the present invention for recombinant human chymase was measured by the method of Pasztor et al. (Pasztor... | US Patent US8846660 (2014) BindingDB Entry DOI: 10.7270/Q22V2DTB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Chymase (Homo sapiens (Human)) | BDBM134265 (US8846660, 37) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 50 | n/a | n/a | n/a | n/a | 7.5 | 25 |
Daiichi Sankyo Company, Ltd. US Patent | Assay Description The inhibitory activity of the compounds of the present invention for recombinant human chymase was measured by the method of Pasztor et al. (Pasztor... | US Patent US8846660 (2014) BindingDB Entry DOI: 10.7270/Q22V2DTB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Chymase (Homo sapiens (Human)) | BDBM134266 (US8846660, 40) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | KEGG PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 10 | n/a | n/a | n/a | n/a | 7.5 | 25 |
Daiichi Sankyo Company, Ltd. US Patent | Assay Description The inhibitory activity of the compounds of the present invention for recombinant human chymase was measured by the method of Pasztor et al. (Pasztor... | US Patent US8846660 (2014) BindingDB Entry DOI: 10.7270/Q22V2DTB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Chymase (Homo sapiens (Human)) | BDBM134267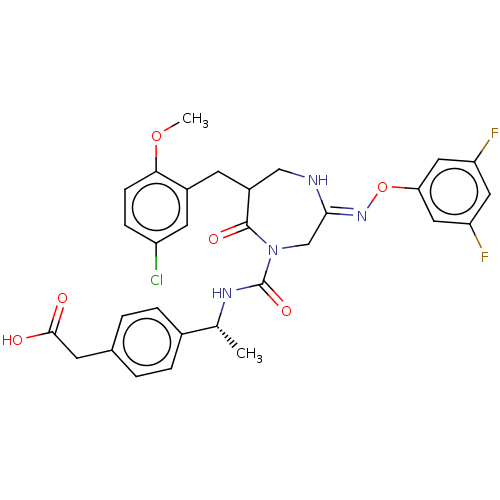 (US8846660, 41) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | KEGG PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 3 | n/a | n/a | n/a | n/a | 7.5 | 25 |
Daiichi Sankyo Company, Ltd. US Patent | Assay Description The inhibitory activity of the compounds of the present invention for recombinant human chymase was measured by the method of Pasztor et al. (Pasztor... | US Patent US8846660 (2014) BindingDB Entry DOI: 10.7270/Q22V2DTB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Chymase (Homo sapiens (Human)) | BDBM134268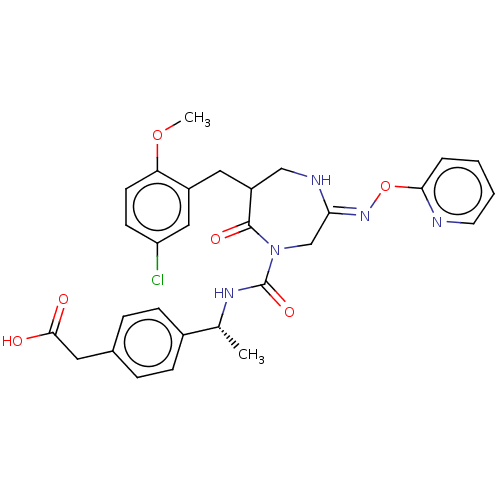 (US8846660, 42) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | KEGG PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 20 | n/a | n/a | n/a | n/a | 7.5 | 25 |
Daiichi Sankyo Company, Ltd. US Patent | Assay Description The inhibitory activity of the compounds of the present invention for recombinant human chymase was measured by the method of Pasztor et al. (Pasztor... | US Patent US8846660 (2014) BindingDB Entry DOI: 10.7270/Q22V2DTB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Chymase (Homo sapiens (Human)) | BDBM134269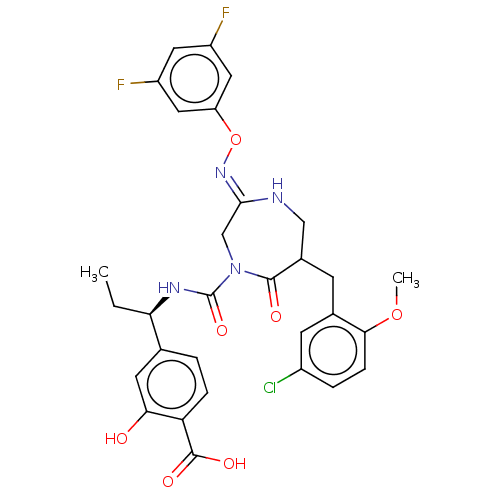 (US8846660, 44) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 20 | n/a | n/a | n/a | n/a | 7.5 | 25 |
Daiichi Sankyo Company, Ltd. US Patent | Assay Description The inhibitory activity of the compounds of the present invention for recombinant human chymase was measured by the method of Pasztor et al. (Pasztor... | US Patent US8846660 (2014) BindingDB Entry DOI: 10.7270/Q22V2DTB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Chymase (Homo sapiens (Human)) | BDBM134270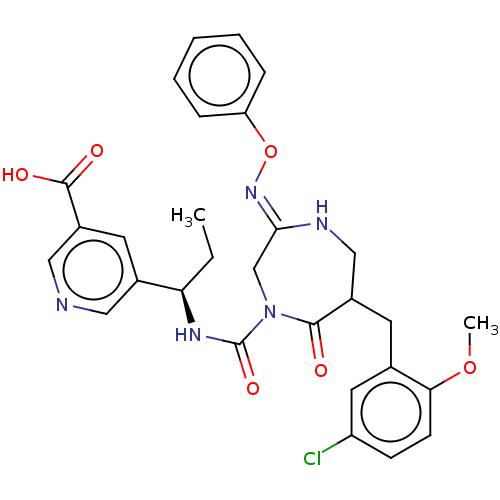 (US8846660, 49) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 30 | n/a | n/a | n/a | n/a | 7.5 | 25 |
Daiichi Sankyo Company, Ltd. US Patent | Assay Description The inhibitory activity of the compounds of the present invention for recombinant human chymase was measured by the method of Pasztor et al. (Pasztor... | US Patent US8846660 (2014) BindingDB Entry DOI: 10.7270/Q22V2DTB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Chymase (Homo sapiens (Human)) | BDBM134271 (US8846660, 53) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 40 | n/a | n/a | n/a | n/a | 7.5 | 25 |
Daiichi Sankyo Company, Ltd. US Patent | Assay Description The inhibitory activity of the compounds of the present invention for recombinant human chymase was measured by the method of Pasztor et al. (Pasztor... | US Patent US8846660 (2014) BindingDB Entry DOI: 10.7270/Q22V2DTB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Chymase (Homo sapiens (Human)) | BDBM134272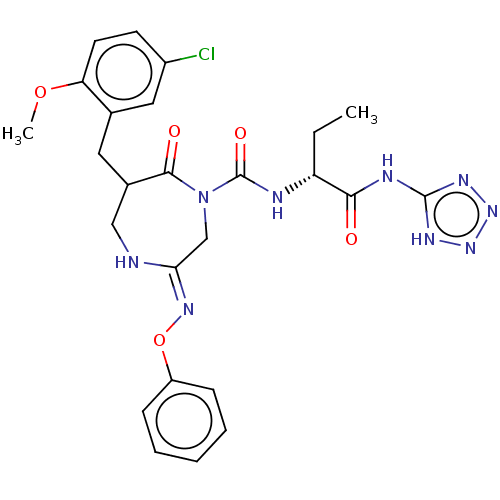 (US8846660, 58) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 20 | n/a | n/a | n/a | n/a | 7.5 | 25 |
Daiichi Sankyo Company, Ltd. US Patent | Assay Description The inhibitory activity of the compounds of the present invention for recombinant human chymase was measured by the method of Pasztor et al. (Pasztor... | US Patent US8846660 (2014) BindingDB Entry DOI: 10.7270/Q22V2DTB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Chymase (Homo sapiens (Human)) | BDBM134273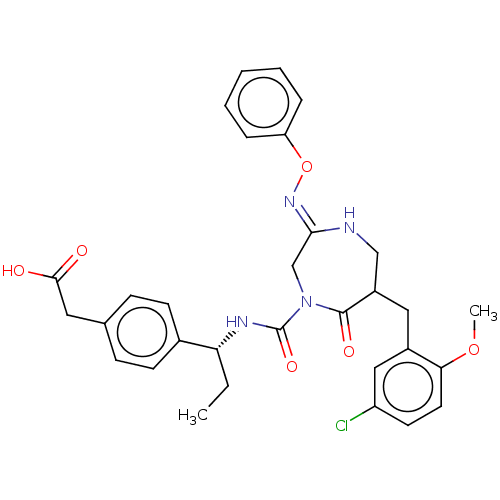 (US8846660, 64) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | KEGG PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 30 | n/a | n/a | n/a | n/a | 7.5 | 25 |
Daiichi Sankyo Company, Ltd. US Patent | Assay Description The inhibitory activity of the compounds of the present invention for recombinant human chymase was measured by the method of Pasztor et al. (Pasztor... | US Patent US8846660 (2014) BindingDB Entry DOI: 10.7270/Q22V2DTB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Chymase (Homo sapiens (Human)) | BDBM134274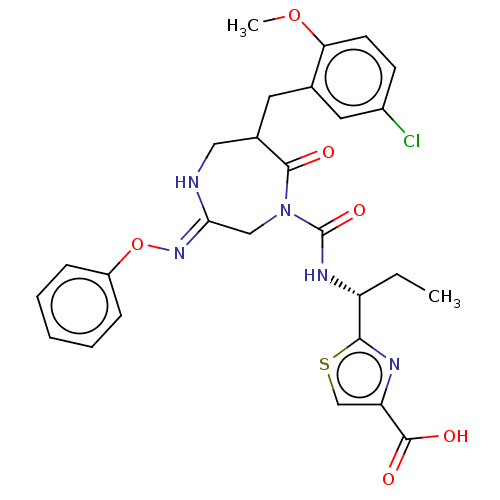 (US8846660, 67) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 50 | n/a | n/a | n/a | n/a | 7.5 | 25 |
Daiichi Sankyo Company, Ltd. US Patent | Assay Description The inhibitory activity of the compounds of the present invention for recombinant human chymase was measured by the method of Pasztor et al. (Pasztor... | US Patent US8846660 (2014) BindingDB Entry DOI: 10.7270/Q22V2DTB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Chymase (Homo sapiens (Human)) | BDBM134275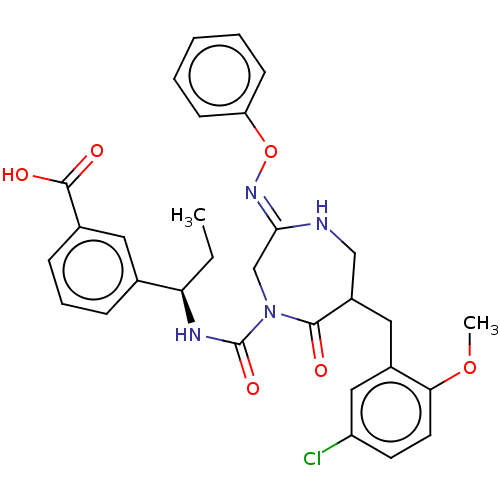 (US8846660, 73) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 30 | n/a | n/a | n/a | n/a | 7.5 | 25 |
Daiichi Sankyo Company, Ltd. US Patent | Assay Description The inhibitory activity of the compounds of the present invention for recombinant human chymase was measured by the method of Pasztor et al. (Pasztor... | US Patent US8846660 (2014) BindingDB Entry DOI: 10.7270/Q22V2DTB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Chymase (Homo sapiens (Human)) | BDBM134276 (US8846660, 76) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 9 | n/a | n/a | n/a | n/a | 7.5 | 25 |
Daiichi Sankyo Company, Ltd. US Patent | Assay Description The inhibitory activity of the compounds of the present invention for recombinant human chymase was measured by the method of Pasztor et al. (Pasztor... | US Patent US8846660 (2014) BindingDB Entry DOI: 10.7270/Q22V2DTB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Chymase (Homo sapiens (Human)) | BDBM134277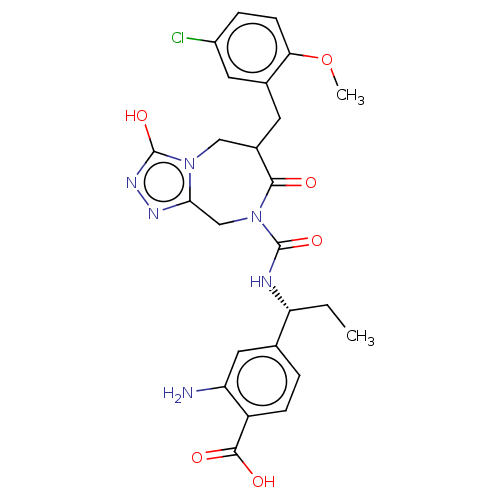 (US8846660, 88) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 60 | n/a | n/a | n/a | n/a | 7.5 | 25 |
Daiichi Sankyo Company, Ltd. US Patent | Assay Description The inhibitory activity of the compounds of the present invention for recombinant human chymase was measured by the method of Pasztor et al. (Pasztor... | US Patent US8846660 (2014) BindingDB Entry DOI: 10.7270/Q22V2DTB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Chymase (Homo sapiens (Human)) | BDBM134278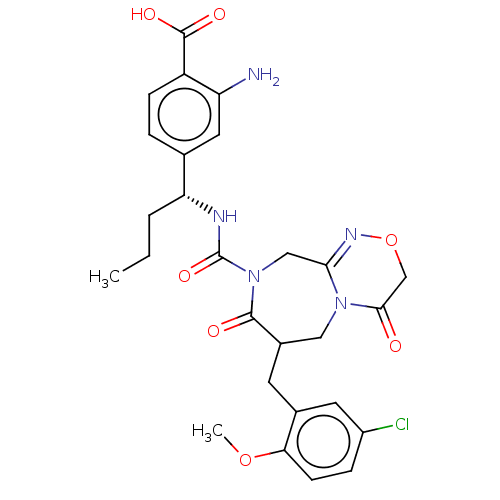 (US8846660, 96) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 7 | n/a | n/a | n/a | n/a | 7.5 | 25 |
Daiichi Sankyo Company, Ltd. US Patent | Assay Description The inhibitory activity of the compounds of the present invention for recombinant human chymase was measured by the method of Pasztor et al. (Pasztor... | US Patent US8846660 (2014) BindingDB Entry DOI: 10.7270/Q22V2DTB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Chymase (Homo sapiens (Human)) | BDBM134280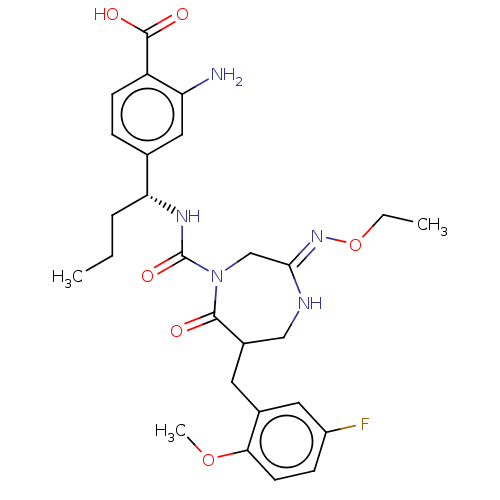 (US8846660, 108) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 30 | n/a | n/a | n/a | n/a | 7.5 | 25 |
Daiichi Sankyo Company, Ltd. US Patent | Assay Description The inhibitory activity of the compounds of the present invention for recombinant human chymase was measured by the method of Pasztor et al. (Pasztor... | US Patent US8846660 (2014) BindingDB Entry DOI: 10.7270/Q22V2DTB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Chymase (Homo sapiens (Human)) | BDBM134253 (US8846660, 103 | US8846660, 12) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 20 | n/a | n/a | n/a | n/a | 7.5 | 25 |
Daiichi Sankyo Company, Ltd. US Patent | Assay Description The inhibitory activity of the compounds of the present invention for recombinant human chymase was measured by the method of Pasztor et al. (Pasztor... | US Patent US8846660 (2014) BindingDB Entry DOI: 10.7270/Q22V2DTB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Chymase (Homo sapiens (Human)) | BDBM100306 (US8501749, 6) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 36 | n/a | n/a | n/a | n/a | 8.0 | 28 |
Boehringer Ingelheim International GmbH US Patent | Assay Description In vitro inhibition assay of Chymase. | US Patent US8501749 (2013) BindingDB Entry DOI: 10.7270/Q28G8JB2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Chymase (Homo sapiens (Human)) | BDBM100307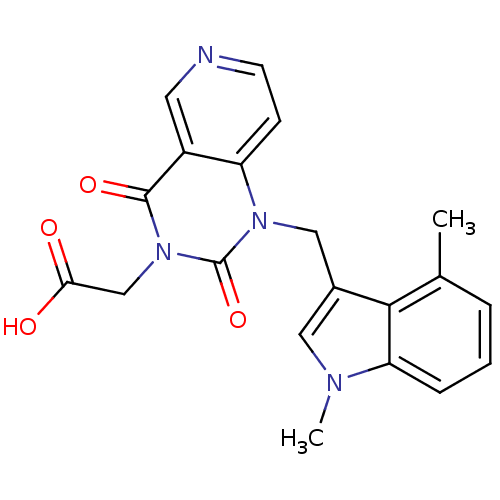 (US8501749, 7) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 9.60 | n/a | n/a | n/a | n/a | 8.0 | 28 |
Boehringer Ingelheim International GmbH US Patent | Assay Description In vitro inhibition assay of Chymase. | US Patent US8501749 (2013) BindingDB Entry DOI: 10.7270/Q28G8JB2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Chymase (Homo sapiens (Human)) | BDBM100308 (US8501749, 8) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 6.10 | n/a | n/a | n/a | n/a | 8.0 | 28 |
Boehringer Ingelheim International GmbH US Patent | Assay Description In vitro inhibition assay of Chymase. | US Patent US8501749 (2013) BindingDB Entry DOI: 10.7270/Q28G8JB2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Chymase (Homo sapiens (Human)) | BDBM100312 (US8501749, 12) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 3.10 | n/a | n/a | n/a | n/a | 8.0 | 28 |
Boehringer Ingelheim International GmbH US Patent | Assay Description In vitro inhibition assay of Chymase. | US Patent US8501749 (2013) BindingDB Entry DOI: 10.7270/Q28G8JB2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Chymase (Homo sapiens (Human)) | BDBM100314 (US8501749, 14) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 11 | n/a | n/a | n/a | n/a | 8.0 | 28 |
Boehringer Ingelheim International GmbH US Patent | Assay Description In vitro inhibition assay of Chymase. | US Patent US8501749 (2013) BindingDB Entry DOI: 10.7270/Q28G8JB2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Chymase (Homo sapiens (Human)) | BDBM100315 (US8501749, 15) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 8 | n/a | n/a | n/a | n/a | 8.0 | 28 |
Boehringer Ingelheim International GmbH US Patent | Assay Description In vitro inhibition assay of Chymase. | US Patent US8501749 (2013) BindingDB Entry DOI: 10.7270/Q28G8JB2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Chymase (Homo sapiens (Human)) | BDBM100316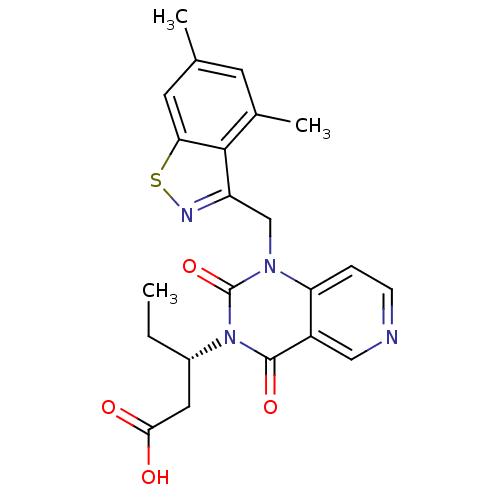 (US8501749, 16) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 17 | n/a | n/a | n/a | n/a | 8.0 | 28 |
Boehringer Ingelheim International GmbH US Patent | Assay Description In vitro inhibition assay of Chymase. | US Patent US8501749 (2013) BindingDB Entry DOI: 10.7270/Q28G8JB2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Chymase (Homo sapiens (Human)) | BDBM100317 (US8501749, 17) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 7 | n/a | n/a | n/a | n/a | 8.0 | 28 |
Boehringer Ingelheim International GmbH US Patent | Assay Description In vitro inhibition assay of Chymase. | US Patent US8501749 (2013) BindingDB Entry DOI: 10.7270/Q28G8JB2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Chymase (Homo sapiens (Human)) | BDBM100318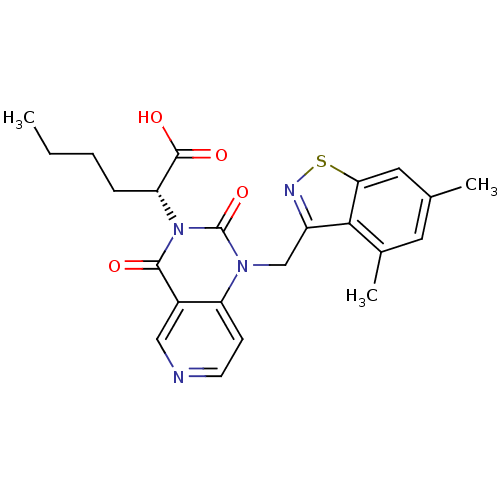 (US8501749, 18) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 2.60 | n/a | n/a | n/a | n/a | 8.0 | 28 |
Boehringer Ingelheim International GmbH US Patent | Assay Description In vitro inhibition assay of Chymase. | US Patent US8501749 (2013) BindingDB Entry DOI: 10.7270/Q28G8JB2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Chymase (Homo sapiens (Human)) | BDBM100319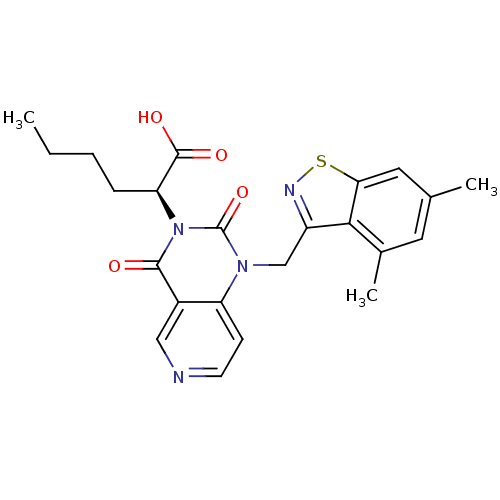 (US8501749, 19) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 7 | n/a | n/a | n/a | n/a | 8.0 | 28 |
Boehringer Ingelheim International GmbH US Patent | Assay Description In vitro inhibition assay of Chymase. | US Patent US8501749 (2013) BindingDB Entry DOI: 10.7270/Q28G8JB2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Chymase (Homo sapiens (Human)) | BDBM100320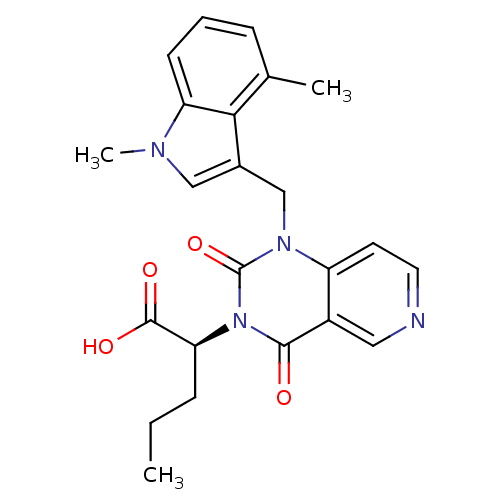 (US8501749, 20) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 2.10 | n/a | n/a | n/a | n/a | 8.0 | 28 |
Boehringer Ingelheim International GmbH US Patent | Assay Description In vitro inhibition assay of Chymase. | US Patent US8501749 (2013) BindingDB Entry DOI: 10.7270/Q28G8JB2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Chymase (Homo sapiens (Human)) | BDBM100321 (US8501749, 21) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 4 | n/a | n/a | n/a | n/a | 8.0 | 28 |
Boehringer Ingelheim International GmbH US Patent | Assay Description In vitro inhibition assay of Chymase. | US Patent US8501749 (2013) BindingDB Entry DOI: 10.7270/Q28G8JB2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Chymase (Homo sapiens (Human)) | BDBM100322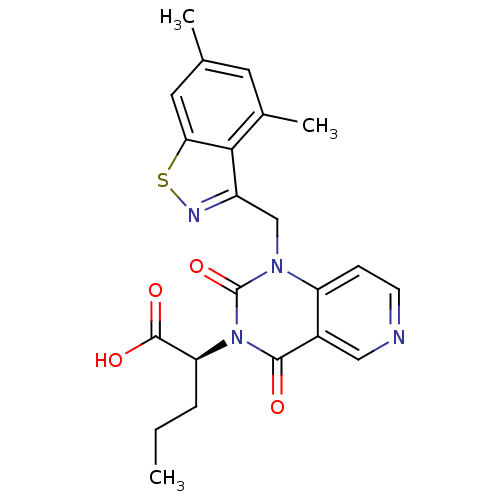 (US8501749, 22) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 4.80 | n/a | n/a | n/a | n/a | 8.0 | 28 |
Boehringer Ingelheim International GmbH US Patent | Assay Description In vitro inhibition assay of Chymase. | US Patent US8501749 (2013) BindingDB Entry DOI: 10.7270/Q28G8JB2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Chymase (Homo sapiens (Human)) | BDBM100324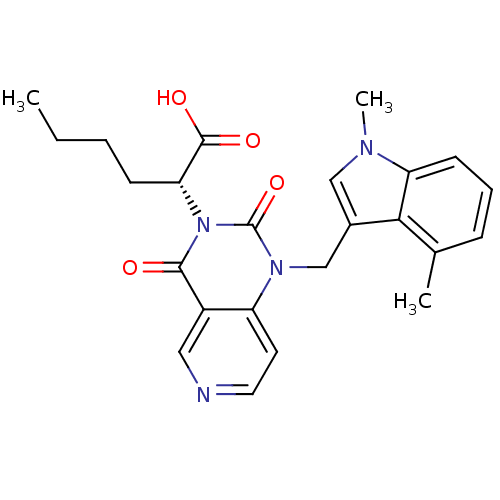 (US8501749, 24) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 0.900 | n/a | n/a | n/a | n/a | 8.0 | 28 |
Boehringer Ingelheim International GmbH US Patent | Assay Description In vitro inhibition assay of Chymase. | US Patent US8501749 (2013) BindingDB Entry DOI: 10.7270/Q28G8JB2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Chymase (Homo sapiens (Human)) | BDBM100325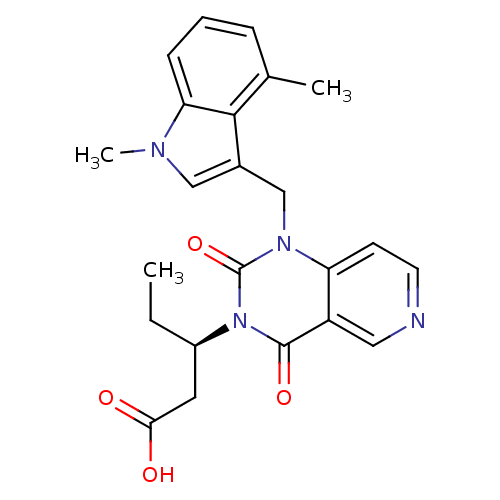 (US8501749, 25) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 2.5 | n/a | n/a | n/a | n/a | 8.0 | 28 |
Boehringer Ingelheim International GmbH US Patent | Assay Description In vitro inhibition assay of Chymase. | US Patent US8501749 (2013) BindingDB Entry DOI: 10.7270/Q28G8JB2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Chymase (Homo sapiens (Human)) | BDBM100326 (US8501749, 26) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 6.10 | n/a | n/a | n/a | n/a | 8.0 | 28 |
Boehringer Ingelheim International GmbH US Patent | Assay Description In vitro inhibition assay of Chymase. | US Patent US8501749 (2013) BindingDB Entry DOI: 10.7270/Q28G8JB2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Chymase (Homo sapiens (Human)) | BDBM100327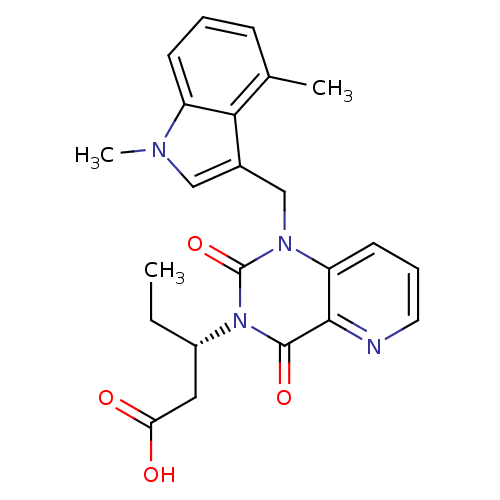 (US8501749, 27) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 3 | n/a | n/a | n/a | n/a | 8.0 | 28 |
Boehringer Ingelheim International GmbH US Patent | Assay Description In vitro inhibition assay of Chymase. | US Patent US8501749 (2013) BindingDB Entry DOI: 10.7270/Q28G8JB2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Chymase (Homo sapiens (Human)) | BDBM100328 (US8501749, 28) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 20 | n/a | n/a | n/a | n/a | 8.0 | 28 |
Boehringer Ingelheim International GmbH US Patent | Assay Description In vitro inhibition assay of Chymase. | US Patent US8501749 (2013) BindingDB Entry DOI: 10.7270/Q28G8JB2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Chymase (Homo sapiens (Human)) | BDBM100329 (US8501749, 29) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 9.90 | n/a | n/a | n/a | n/a | 8.0 | 28 |
Boehringer Ingelheim International GmbH US Patent | Assay Description In vitro inhibition assay of Chymase. | US Patent US8501749 (2013) BindingDB Entry DOI: 10.7270/Q28G8JB2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Chymase (Homo sapiens (Human)) | BDBM100330 (US8501749, 30) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 1.40 | n/a | n/a | n/a | n/a | 8.0 | 28 |
Boehringer Ingelheim International GmbH US Patent | Assay Description In vitro inhibition assay of Chymase. | US Patent US8501749 (2013) BindingDB Entry DOI: 10.7270/Q28G8JB2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Chymase (Homo sapiens (Human)) | BDBM100331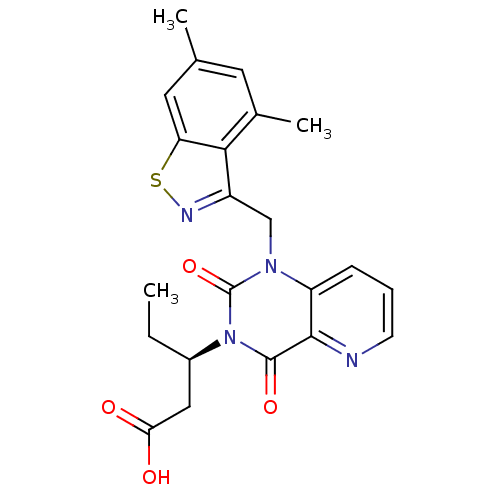 (US8501749, 31) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 2.20 | n/a | n/a | n/a | n/a | 8.0 | 28 |
Boehringer Ingelheim International GmbH US Patent | Assay Description In vitro inhibition assay of Chymase. | US Patent US8501749 (2013) BindingDB Entry DOI: 10.7270/Q28G8JB2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 71 total ) | Next | Last >> |