

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| P2X purinoceptor 1 (RAT) | BDBM50118215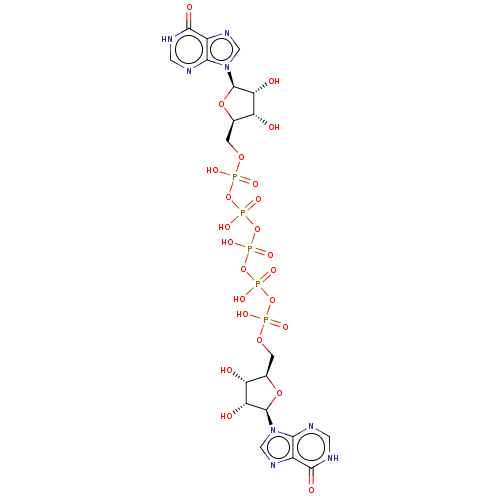 (CHEMBL1628528 | Ip5I) | Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | n/a | n/a | 1.5 | n/a | n/a | n/a | n/a |
National Institute of Diabetes Curated by ChEMBL | Assay Description The compound was evaluated for antagonist activity against recombinant rat P2X purinoceptor 1 (P2X1) | J Med Chem 45: 4057-93 (2002) BindingDB Entry DOI: 10.7270/Q2VX0H71 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| P2X purinoceptor 1 (RAT) | BDBM50118222 (MRS 2257) | Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem Similars | PubMed | n/a | n/a | n/a | n/a | 5 | n/a | n/a | n/a | n/a |
National Institute of Diabetes Curated by ChEMBL | Assay Description The compound was evaluated for antagonist activity against recombinant rat P2X purinoceptor 1 (P2X1) at 1 uM,expressed in Xenopus oocytes | J Med Chem 45: 4057-93 (2002) BindingDB Entry DOI: 10.7270/Q2VX0H71 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| P2X purinoceptor 1 (RAT) | BDBM50118244 (CHEMBL131091 | PPNDS) | Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL MMDB PC cid PC sid UniChem Similars | PubMed | n/a | n/a | n/a | n/a | 14 | n/a | n/a | n/a | n/a |
National Institute of Diabetes Curated by ChEMBL | Assay Description The compound was evaluated for antagonist activity against recombinant rat P2X purinoceptor 1 (P2X1) | J Med Chem 45: 4057-93 (2002) BindingDB Entry DOI: 10.7270/Q2VX0H71 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| P2X purinoceptor 1 (RAT) | BDBM50064800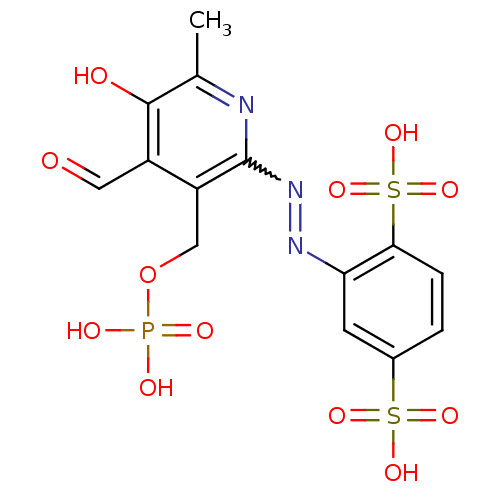 (2-(4-Formyl-5-hydroxy-6-methyl-3-phosphonooxymethy...) | Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | n/a | n/a | 43 | n/a | n/a | n/a | n/a |
National Institute of Diabetes Curated by ChEMBL | Assay Description The compound was evaluated for antagonist activity against recombinant rat P2X purinoceptor 1 (P2X1) | J Med Chem 45: 4057-93 (2002) BindingDB Entry DOI: 10.7270/Q2VX0H71 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| P2X purinoceptor 1 (RAT) | BDBM50118226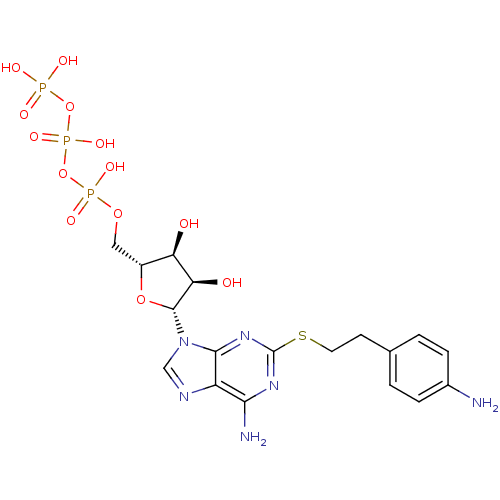 (CHEMBL337062 | PAPET-ATP) | Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | PubMed | n/a | n/a | n/a | n/a | 98 | n/a | n/a | n/a | n/a |
National Institute of Diabetes Curated by ChEMBL | Assay Description The compound was evaluated for antagonist activity against recombinant rat P2X purinoceptor 1 (P2X1) at 1 uM,expressed in Xenopus oocytes | J Med Chem 45: 4057-93 (2002) BindingDB Entry DOI: 10.7270/Q2VX0H71 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| P2X purinoceptor 1 (RAT) | BDBM85043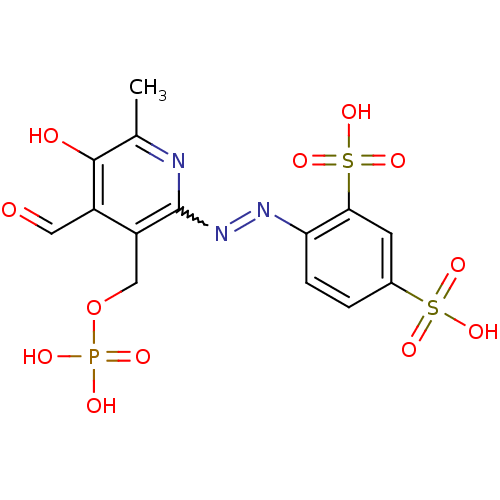 (CAS_149017-66-3 | CHEMBL69234 | NSC_6093163 | PPAD...) | Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid PDB UniChem Similars | PubMed | n/a | n/a | n/a | n/a | 99 | n/a | n/a | n/a | n/a |
National Institute of Diabetes Curated by ChEMBL | Assay Description The compound was evaluated for antagonist activity against recombinant rat P2X purinoceptor 1 (P2X1) at 1 uM,expressed in Xenopus oocytes | J Med Chem 45: 4057-93 (2002) BindingDB Entry DOI: 10.7270/Q2VX0H71 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| P2X purinoceptor 1 (RAT) | BDBM50118240 (((2R,3S,4R,5R)-5-(6-amino-2-(hexylthio)-9H-purin-9...) | Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | n/a | n/a | 840 | n/a | n/a | n/a | n/a |
National Institute of Diabetes Curated by ChEMBL | Assay Description The compound was evaluated for antagonist activity against recombinant rat P2X purinoceptor 1 (P2X1) at 1 uM,expressed in Xenopus oocytes | J Med Chem 45: 4057-93 (2002) BindingDB Entry DOI: 10.7270/Q2VX0H71 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| P2X purinoceptor 1 (RAT) | BDBM50336799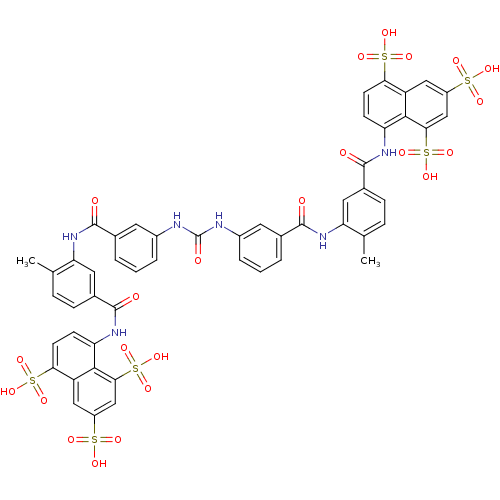 (5,5',5''-[1,3,6-naphthalenetriyltris(sulfonylimino...) | Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG PC cid PC sid PDB UniChem Patents Similars | PubMed | n/a | n/a | n/a | n/a | 1.00E+3 | n/a | n/a | n/a | n/a |
National Institute of Diabetes Curated by ChEMBL | Assay Description The compound was evaluated for antagonist activity against recombinant rat P2X purinoceptor 1 (P2X1) at 1 uM,expressed in Xenopus oocytes | J Med Chem 45: 4057-93 (2002) BindingDB Entry DOI: 10.7270/Q2VX0H71 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| P2X purinoceptor 1 (RAT) | BDBM50118216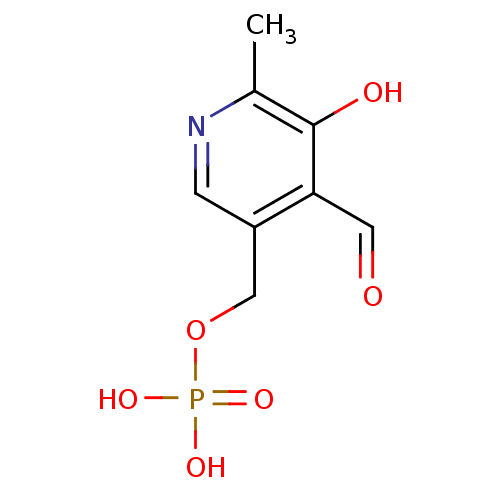 ((4-formyl-5-hydroxy-6-methylpyridin-3-yl)methyl di...) | Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | PubMed | n/a | n/a | n/a | n/a | 3.00E+3 | n/a | n/a | n/a | n/a |
National Institute of Diabetes Curated by ChEMBL | Assay Description The compound was evaluated for antagonist activity against recombinant rat P2X purinoceptor 1 (P2X1) | J Med Chem 45: 4057-93 (2002) BindingDB Entry DOI: 10.7270/Q2VX0H71 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| P2X purinoceptor 1 (RAT) | BDBM50064803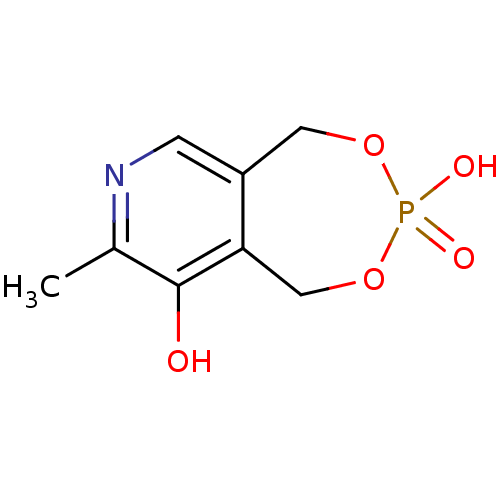 (3-Methyl-7-oxo-5,9-dihydro-6,8-dioxa-2-aza-7lambda...) | Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | n/a | n/a | 5.90E+3 | n/a | n/a | n/a | n/a |
National Institute of Diabetes and Digestive and Kidney Diseases Curated by ChEMBL | Assay Description Compound was tested in a functional ion channel assay of ATP-induced current at recombinant rat P2X1 receptor expressed in Xenopus oocytes. | J Med Chem 41: 2201-6 (1998) Article DOI: 10.1021/jm980183o BindingDB Entry DOI: 10.7270/Q2DB80ZC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| P2X purinoceptor 1 (RAT) | BDBM50064803 (3-Methyl-7-oxo-5,9-dihydro-6,8-dioxa-2-aza-7lambda...) | Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem | PubMed | n/a | n/a | n/a | n/a | 5.90E+3 | n/a | n/a | n/a | n/a |
National Institute of Diabetes and Digestive and Kidney Diseases Curated by ChEMBL | Assay Description Inhibition of inward ion current elicited by ATP at P2X1 receptor expressed in Xenopus oocytes | J Med Chem 44: 340-9 (2001) BindingDB Entry DOI: 10.7270/Q2FN15HC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| P2X purinoceptor 1 (RAT) | BDBM50064803 (3-Methyl-7-oxo-5,9-dihydro-6,8-dioxa-2-aza-7lambda...) | Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem | PubMed | n/a | n/a | n/a | n/a | 1.02E+4 | n/a | n/a | n/a | n/a |
National Institute of Diabetes Curated by ChEMBL | Assay Description The compound was evaluated for antagonist activity against recombinant rat P2X purinoceptor 1 (P2X1) | J Med Chem 45: 4057-93 (2002) BindingDB Entry DOI: 10.7270/Q2VX0H71 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||