

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 5-hydroxytryptamine receptor 1D (Mus musculus (Mouse)) | BDBM86708 (CAS_146714-97-8 | CHEMBL31354 | CHEMBL514874 | CHE...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Similars | Article PubMed | 0.340 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Institutes of Health Curated by ChEMBL | Assay Description Receptor-linked G protein activation at 5-hydroxytryptamine receptor was determined by measuring the stimulation of [35S]-GTP-gammaS, binding (Experi... | J Med Chem 42: 1576-86 (1999) Article DOI: 10.1021/jm980456f BindingDB Entry DOI: 10.7270/Q23R0S2J | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 1D (Mus musculus (Mouse)) | BDBM50076428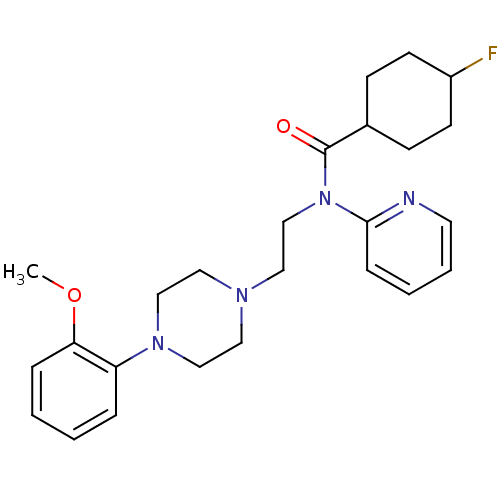 (4-Fluoro-cyclohexanecarboxylic acid {2-[4-(2-metho...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Institutes of Health Curated by ChEMBL | Assay Description Receptor-linked G protein activation at 5-hydroxytryptamine receptor was determined by measuring the stimulation of [35S]-GTP-gammaS, binding | J Med Chem 42: 1576-86 (1999) Article DOI: 10.1021/jm980456f BindingDB Entry DOI: 10.7270/Q23R0S2J | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 1D (Mus musculus (Mouse)) | BDBM86708 (CAS_146714-97-8 | CHEMBL31354 | CHEMBL514874 | CHE...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Similars | Article PubMed | 1.10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Institutes of Health Curated by ChEMBL | Assay Description Receptor-linked G protein activation at 5-hydroxytryptamine receptor was determined by measuring the stimulation of [35S]GTP-gamma-S, binding (Experi... | J Med Chem 42: 1576-86 (1999) Article DOI: 10.1021/jm980456f BindingDB Entry DOI: 10.7270/Q23R0S2J | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 1D (Mus musculus (Mouse)) | BDBM50076429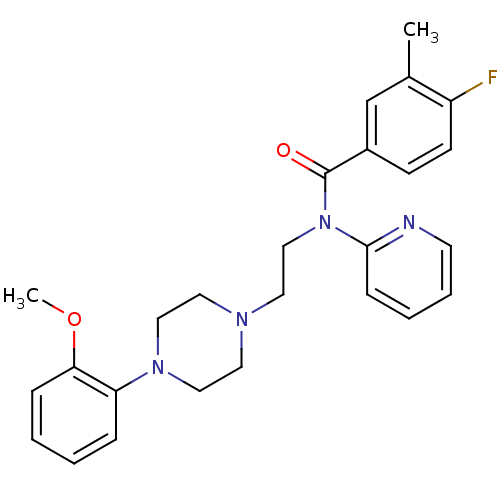 (4-Fluoro-N-{2-[4-(2-methoxy-phenyl)-piperazin-1-yl...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 2.90 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Institutes of Health Curated by ChEMBL | Assay Description Receptor-linked G protein activation at 5-hydroxytryptamine receptor was determined by measuring the stimulation of [35S]-GTP-gammaS, binding (Experi... | J Med Chem 42: 1576-86 (1999) Article DOI: 10.1021/jm980456f BindingDB Entry DOI: 10.7270/Q23R0S2J | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 1D (Mus musculus (Mouse)) | BDBM50076429 (4-Fluoro-N-{2-[4-(2-methoxy-phenyl)-piperazin-1-yl...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Institutes of Health Curated by ChEMBL | Assay Description Receptor-linked G protein activation at 5-hydroxytryptamine receptor was determined by measuring the stimulation of [35S]GTP-gamma-S, binding | J Med Chem 42: 1576-86 (1999) Article DOI: 10.1021/jm980456f BindingDB Entry DOI: 10.7270/Q23R0S2J | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 1D (Mus musculus (Mouse)) | BDBM186927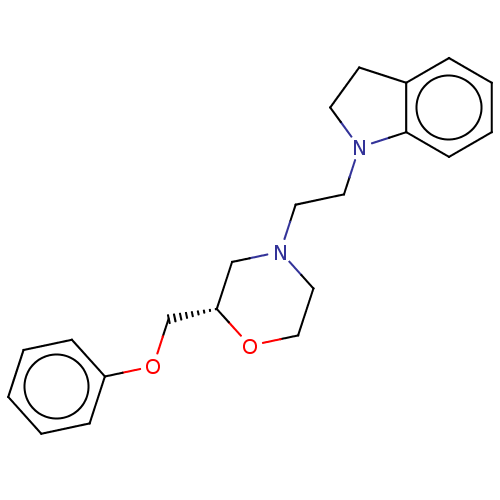 (US9079895, 19s) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | 590 | n/a | n/a | n/a | n/a | n/a | n/a | 7.4 | n/a |
University of Dundee US Patent | Assay Description The detailed experimental protocols for the radioligand and functional receptor assays are available on the NIMH PDSP website at http://pdsp.med.un... | US Patent US9079895 (2015) BindingDB Entry DOI: 10.7270/Q2D7996K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 1D (Mus musculus (Mouse)) | BDBM186935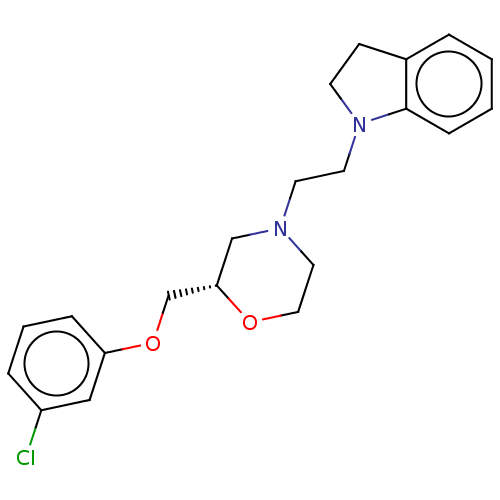 (US9079895, 23s) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | 1.12E+3 | n/a | n/a | n/a | n/a | n/a | n/a | 7.4 | n/a |
University of Dundee US Patent | Assay Description The detailed experimental protocols for the radioligand and functional receptor assays are available on the NIMH PDSP website at http://pdsp.med.un... | US Patent US9079895 (2015) BindingDB Entry DOI: 10.7270/Q2D7996K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 1D (Mus musculus (Mouse)) | BDBM186937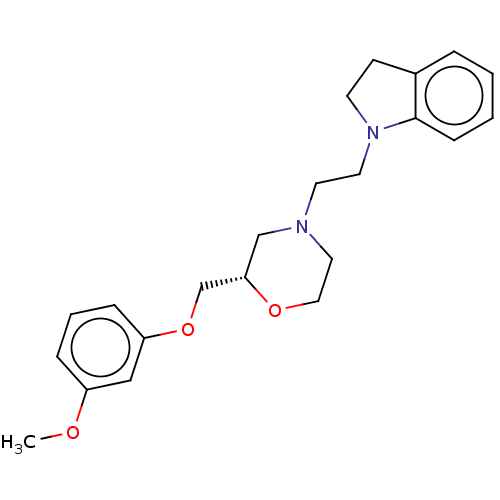 (US9079895, 25s) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | 1.98E+3 | n/a | n/a | n/a | n/a | n/a | n/a | 7.4 | n/a |
University of Dundee US Patent | Assay Description The detailed experimental protocols for the radioligand and functional receptor assays are available on the NIMH PDSP website at http://pdsp.med.un... | US Patent US9079895 (2015) BindingDB Entry DOI: 10.7270/Q2D7996K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 1D (Mus musculus (Mouse)) | BDBM79215 (CHEMBL15928 | GR 127935 | GR 127935 hydrochloride ...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Similars | Article PubMed | <1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Glaxo Research and Development Ltd. Curated by ChEMBL | Assay Description Tested for 5-hydroxytryptamine receptor uptake | J Med Chem 37: 2253-7 (1994) Article DOI: 10.1021/jm00041a001 BindingDB Entry DOI: 10.7270/Q29889QB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||