 Found 24 hits Enz. Inhib. hit(s) with Target = 'Transcription initiation factor TFIID subunit 1-like'
Found 24 hits Enz. Inhib. hit(s) with Target = 'Transcription initiation factor TFIID subunit 1-like' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Transcription initiation factor TFIID subunit 1-like
(Homo sapiens (Human)) | BDBM50539801
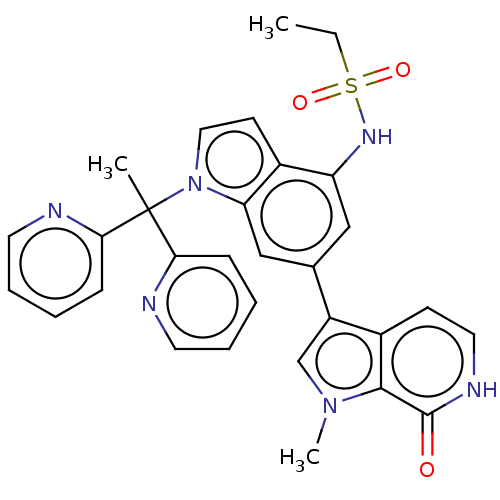
(CHEMBL4648912 | US11840533, Compound 70)Show SMILES CCS(=O)(=O)Nc1cc(cc2n(ccc12)C(C)(c1ccccn1)c1ccccn1)-c1cn(C)c2c1cc[nH]c2=O Show InChI InChI=1S/C30H28N6O3S/c1-4-40(38,39)34-24-17-20(23-19-35(3)28-21(23)11-15-33-29(28)37)18-25-22(24)12-16-36(25)30(2,26-9-5-7-13-31-26)27-10-6-8-14-32-27/h5-19,34H,4H2,1-3H3,(H,33,37) | PDB
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
PDB
UniChem
| Article
PubMed
| n/a | n/a | n/a | <1 | n/a | n/a | n/a | n/a | n/a |
University of Illinois at Chicago
Curated by ChEMBL
| Assay Description
Binding affinity to human partial length TAF1L bromodomain 2 (Q1523 to D1654 residues) expressed in bacterial expression system by BROMOscan assay |
J Med Chem 63: 7186-7210 (2020)
Article DOI: 10.1021/acs.jmedchem.0c00456
BindingDB Entry DOI: 10.7270/Q2PK0KPP |
More data for this
Ligand-Target Pair | |
Transcription initiation factor TFIID subunit 1-like
(Homo sapiens (Human)) | BDBM50503406
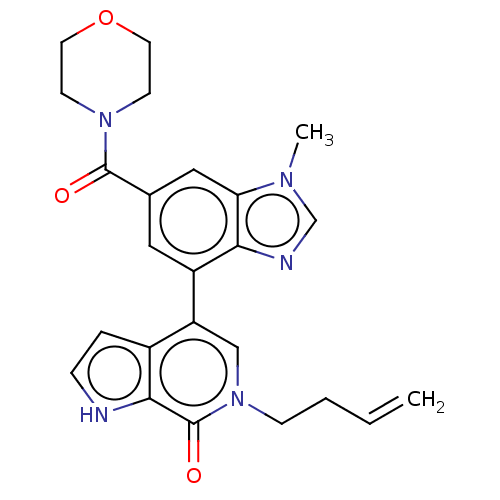
(CHEMBL4463538)Show SMILES Cn1cnc2c(cc(cc12)C(=O)N1CCOCC1)-c1cn(CCC=C)c(=O)c2[nH]ccc12 Show InChI InChI=1S/C24H25N5O3/c1-3-4-7-29-14-19(17-5-6-25-22(17)24(29)31)18-12-16(13-20-21(18)26-15-27(20)2)23(30)28-8-10-32-11-9-28/h3,5-6,12-15,25H,1,4,7-11H2,2H3 | PDB
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
PDB
UniChem
| PDB
Article
PubMed
| n/a | n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a |
Genentech Inc.
Curated by ChEMBL
| Assay Description
Binding affinity to recombinant human full length DNA-tagged TAF1L bromodomain 2 (1523 to 1654 residues) expressed in bacterial expression system by ... |
J Med Chem 61: 9301-9315 (2018)
Article DOI: 10.1021/acs.jmedchem.8b01225
BindingDB Entry DOI: 10.7270/Q2NV9NH4 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Transcription initiation factor TFIID subunit 1-like
(Homo sapiens (Human)) | BDBM50514857

(CHEMBL4449894)Show SMILES C[C@H]1CCN(C1)S(=O)(=O)c1cc(NC(=O)CN2C(=O)N[C@@](C)(C3CC3)C2=O)ccc1Br |r| Show InChI InChI=1S/C20H25BrN4O5S/c1-12-7-8-24(10-12)31(29,30)16-9-14(5-6-15(16)21)22-17(26)11-25-18(27)20(2,13-3-4-13)23-19(25)28/h5-6,9,12-13H,3-4,7-8,10-11H2,1-2H3,(H,22,26)(H,23,28)/t12-,20-/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | n/a | 25 | n/a | n/a | n/a | n/a | n/a |
University of Strathclyde
Curated by ChEMBL
| Assay Description
Binding affinity to human partial length TAF1L bromodomain 2 (Q1523 to D1654 residues) expressed in bacterial expression system by BROMOscan assay |
J Med Chem 63: 5212-5241 (2020)
Article DOI: 10.1021/acs.jmedchem.0c00021
BindingDB Entry DOI: 10.7270/Q2RN3C7T |
More data for this
Ligand-Target Pair | |
Transcription initiation factor TFIID subunit 1-like
(Homo sapiens (Human)) | BDBM50560174
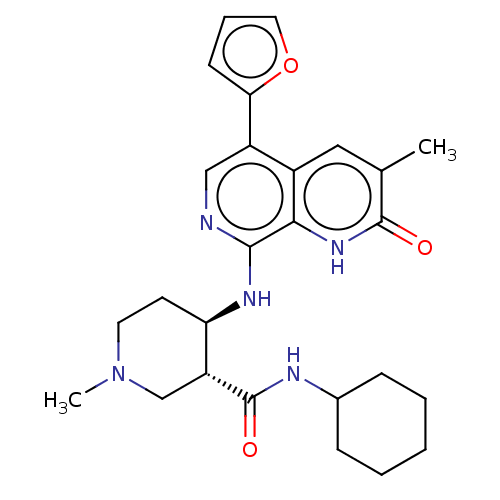
(GSK789)Show SMILES CN1CC[C@@H](Nc2ncc(-c3ccco3)c3cc(C)c(=O)[nH]c23)[C@@H](C1)C(=O)NC1CCCCC1 | PDB
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| MCE
PC cid
PC sid
PDB
UniChem
| Article
PubMed
| n/a | n/a | n/a | 398 | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Binding affinity to human partial length TAF1L bromodomain 2 (Q1523 to D1654 residues) expressed in bacterial expression system by BROMOscan assay |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.0c00614
BindingDB Entry DOI: 10.7270/Q27D2ZVG |
More data for this
Ligand-Target Pair | |
Transcription initiation factor TFIID subunit 1-like
(Homo sapiens (Human)) | BDBM50615276
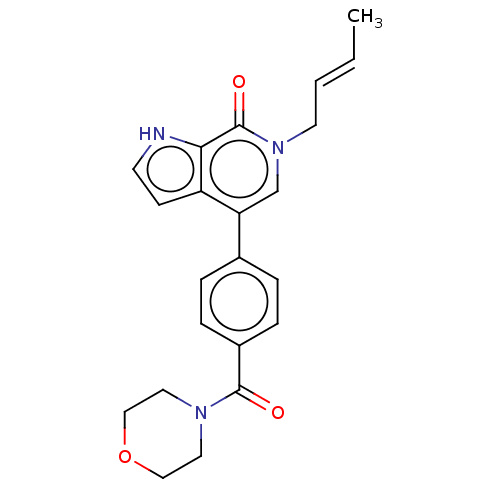
(CHEMBL5270178) | PDB
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| UniChem
| | n/a | n/a | n/a | 610 | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Transcription initiation factor TFIID subunit 1-like
(Homo sapiens (Human)) | BDBM220447

(US10633379, Compound X | US9296741, 36)Show SMILES CCS(=O)(=O)Nc1ccc(Oc2ccc(F)cc2F)c(c1)-c1cn(C)c(=O)c2[nH]ccc12 Show InChI InChI=1S/C22H19F2N3O4S/c1-3-32(29,30)26-14-5-7-19(31-20-6-4-13(23)10-18(20)24)16(11-14)17-12-27(2)22(28)21-15(17)8-9-25-21/h4-12,25-26H,3H2,1-2H3 | PDB
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
PDB
UniChem
| Article
PubMed
| n/a | n/a | n/a | >1.00E+3 | n/a | n/a | n/a | n/a | n/a |
AbbVie Inc.
Curated by ChEMBL
| Assay Description
Binding affinity to human TAF1L (2) (Q1523 to D1654 residues) expressed in bacterial expression system by BROMOscan assay |
J Med Chem 60: 8369-8384 (2017)
Article DOI: 10.1021/acs.jmedchem.7b00746
BindingDB Entry DOI: 10.7270/Q2251MB8 |
More data for this
Ligand-Target Pair | |
Transcription initiation factor TFIID subunit 1-like
(Homo sapiens (Human)) | BDBM50030894
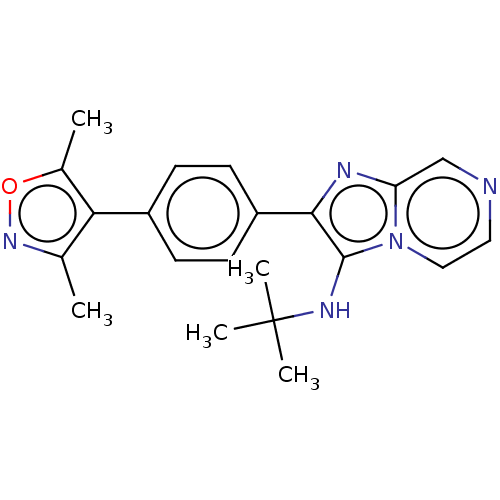
(CHEMBL3356559)Show SMILES Cc1noc(C)c1-c1ccc(cc1)-c1nc2cnccn2c1NC(C)(C)C Show InChI InChI=1S/C21H23N5O/c1-13-18(14(2)27-25-13)15-6-8-16(9-7-15)19-20(24-21(3,4)5)26-11-10-22-12-17(26)23-19/h6-12,24H,1-5H3 | PDB
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
PDB
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | 1.30E+3 | n/a | n/a | n/a | n/a | n/a |
Dana-Farber Institute
Curated by ChEMBL
| Assay Description
Binding affinity to TAF1L (unknown origin) by bromodomain displacement assay |
J Med Chem 57: 9019-27 (2014)
Article DOI: 10.1021/jm501120z
BindingDB Entry DOI: 10.7270/Q2FN17T7 |
More data for this
Ligand-Target Pair | |
Transcription initiation factor TFIID subunit 1-like
(Homo sapiens (Human)) | BDBM50148270

(CHEMBL3739699)Show SMILES CCCOc1ccn2c(cc(-c3ccccc3S(C)(=O)=O)c2c1)C(C)=O Show InChI InChI=1S/C20H21NO4S/c1-4-11-25-15-9-10-21-18(14(2)22)13-17(19(21)12-15)16-7-5-6-8-20(16)26(3,23)24/h5-10,12-13H,4,11H2,1-3H3 | PDB
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
PDB
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | 3.19E+3 | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Binding affinity to biotinylated C-terminal Avi/His-TEV-fused TAF1L bromodomain-2 (unknown origin) expressed in Escherichia coli by isothermal titrat... |
J Med Chem 59: 1410-24 (2016)
Article DOI: 10.1021/acs.jmedchem.5b00209
BindingDB Entry DOI: 10.7270/Q2PK0J09 |
More data for this
Ligand-Target Pair | |
Transcription initiation factor TFIID subunit 1-like
(Homo sapiens (Human)) | BDBM50453949

(CHEMBL4208820 | US11247989, Example 87)Show SMILES CNC(=O)c1ccc(cn1)-c1cc2cccc(-c3nn(C4CCOCC4)c4CCN(Cc34)C(C)=O)c2cn1 Show InChI InChI=1S/C29H30N6O3/c1-18(36)34-11-8-27-24(17-34)28(33-35(27)21-9-12-38-13-10-21)22-5-3-4-19-14-26(32-16-23(19)22)20-6-7-25(31-15-20)29(37)30-2/h3-7,14-16,21H,8-13,17H2,1-2H3,(H,30,37) | PDB
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | n/a | 3.20E+3 | n/a | n/a | n/a | n/a | n/a |
WuXi AppTec Co., Ltd.
Curated by ChEMBL
| Assay Description
Binding affinity to human partial length TAF1L bromodomain 2 (Q1523 to D1654 residues) expressed in bacterial expression system by BROMOscan assay |
Bioorg Med Chem Lett 28: 15-23 (2018)
Article DOI: 10.1016/j.bmcl.2017.11.025
BindingDB Entry DOI: 10.7270/Q2ZG6VVZ |
More data for this
Ligand-Target Pair | |
Transcription initiation factor TFIID subunit 1-like
(Homo sapiens (Human)) | BDBM50366670
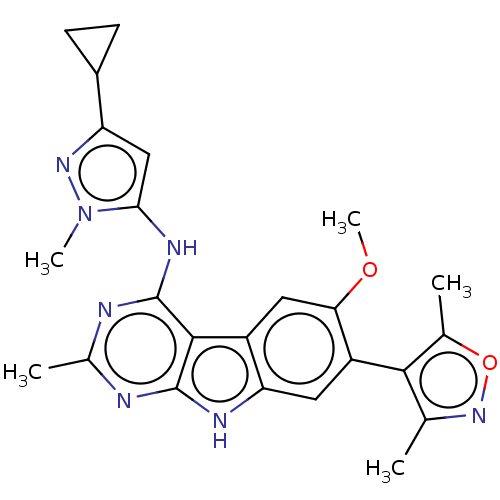
(CHEMBL4173488)Show SMILES COc1cc2c(cc1-c1c(C)noc1C)[nH]c1nc(C)nc(Nc3cc(nn3C)C3CC3)c21 |(86.87,-46.33,;85.61,-45.43,;84.21,-46.07,;84.06,-47.6,;82.66,-48.24,;81.41,-47.34,;81.56,-45.82,;82.95,-45.18,;83.09,-43.65,;81.94,-42.63,;80.43,-42.97,;82.55,-41.22,;84.08,-41.36,;84.42,-42.87,;85.83,-43.48,;80.17,-48.26,;80.66,-49.73,;79.89,-51.06,;80.66,-52.39,;79.89,-53.73,;82.21,-52.4,;82.97,-51.06,;84.51,-51.06,;85.28,-52.39,;84.66,-53.8,;85.81,-54.83,;87.14,-54.06,;86.82,-52.56,;87.85,-51.41,;85.64,-56.36,;84.75,-57.61,;86.28,-57.77,;82.2,-49.71,)| Show InChI InChI=1S/C24H25N7O2/c1-11-21(12(2)33-30-11)16-8-18-15(9-19(16)32-5)22-23(27-18)25-13(3)26-24(22)28-20-10-17(14-6-7-14)29-31(20)4/h8-10,14H,6-7H2,1-5H3,(H2,25,26,27,28) | PDB
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
PDB
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | >5.00E+3 | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Binding affinity to human TAF1L bromodomain 2 (Q1523 to D1654 residues) expressed in bacterial expression system by BROMOScan assay |
J Med Chem 61: 6110-6120 (2018)
Article DOI: 10.1021/acs.jmedchem.8b00483
BindingDB Entry DOI: 10.7270/Q26M39B1 |
More data for this
Ligand-Target Pair | |
Transcription initiation factor TFIID subunit 1-like
(Homo sapiens (Human)) | BDBM321424
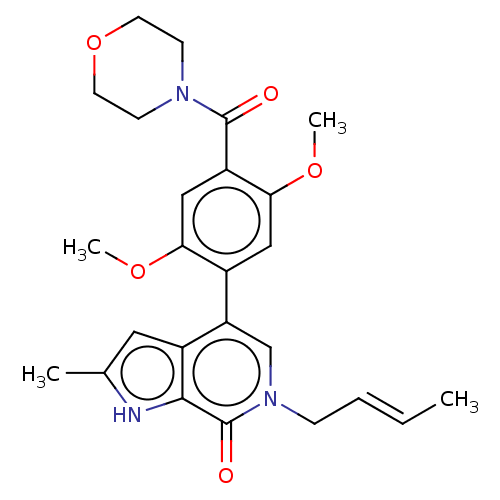
(6-[(E)-but-2-enyl]-4-[2,5-dimethoxy-4-(morpholine-...)Show SMILES COc1cc(c(OC)cc1C(=O)N1CCOCC1)-c1cn(C\C=C\C)c(=O)c2[nH]c(C)cc12 Show InChI InChI=1S/C25H29N3O5/c1-5-6-7-28-15-20(18-12-16(2)26-23(18)25(28)30)17-13-22(32-4)19(14-21(17)31-3)24(29)27-8-10-33-11-9-27/h5-6,12-15,26H,7-11H2,1-4H3/b6-5+ | PDB
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
| | n/a | n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Transcription initiation factor TFIID subunit 1-like
(Homo sapiens (Human)) | BDBM50615275
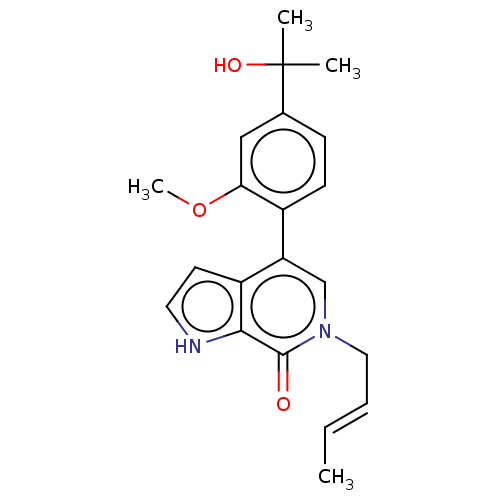
(CHEMBL5282240) | PDB
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| UniChem
| | n/a | n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Transcription initiation factor TFIID subunit 1-like
(Homo sapiens (Human)) | BDBM321437
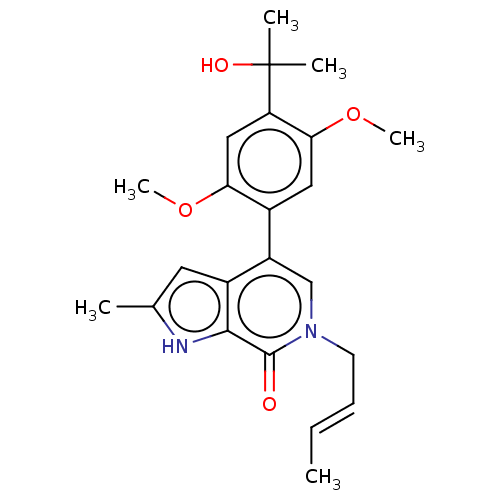
((E)-6-(but-2-en-1-yl)-4-(4-(2-hydroxypropan-2-yl)-...)Show SMILES COc1cc(c(OC)cc1-c1cn(C\C=C\C)c(=O)c2[nH]c(C)cc12)C(C)(C)O Show InChI InChI=1S/C23H28N2O4/c1-7-8-9-25-13-17(16-10-14(2)24-21(16)22(25)26)15-11-20(29-6)18(23(3,4)27)12-19(15)28-5/h7-8,10-13,24,27H,9H2,1-6H3/b8-7+ | PDB
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | n/a | n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Transcription initiation factor TFIID subunit 1-like
(Homo sapiens (Human)) | BDBM249277
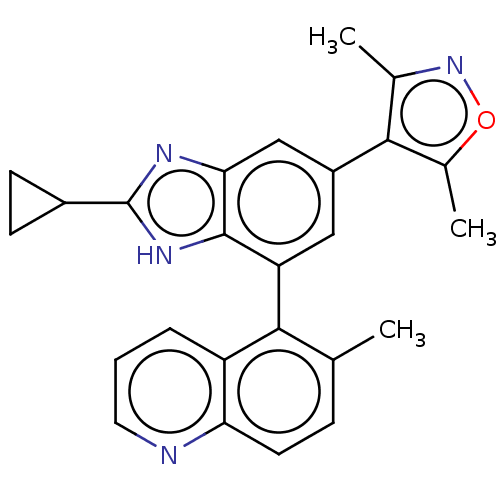
(US10017501, Compound 1020-18 | US9458145, 1020-18)Show SMILES Cc1noc(C)c1-c1cc(-c2c(C)ccc3ncccc23)c2[nH]c(nc2c1)C1CC1 |(1.89,3.57,;.56,4.34,;.08,5.8,;-1.46,5.8,;-1.94,4.34,;-3.27,3.57,;-.69,3.44,;-.69,1.9,;.64,1.13,;.64,-.41,;1.98,-1.18,;3.31,-.41,;3.31,1.13,;4.65,-1.18,;4.65,-2.72,;3.31,-3.49,;3.31,-5.03,;1.98,-5.8,;.64,-5.03,;.64,-3.49,;1.98,-2.72,;-.69,-1.18,;-1.01,-2.69,;-2.54,-2.85,;-3.17,-1.45,;-2.02,-.41,;-2.02,1.13,;-3.31,-4.19,;-4.65,-4.96,;-3.31,-5.73,)| Show InChI InChI=1S/C25H22N4O/c1-13-6-9-20-18(5-4-10-26-20)22(13)19-11-17(23-14(2)29-30-15(23)3)12-21-24(19)28-25(27-21)16-7-8-16/h4-6,9-12,16H,7-8H2,1-3H3,(H,27,28) | PDB
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a |
Gilead Sciences, Inc.
Curated by ChEMBL
| Assay Description
Binding affinity to human partial length TAF1L bromodomain 2 (Q1523 to D1654 residues) expressed in bacterial expression system after 1 hr by bromosc... |
Bioorg Med Chem 27: 457-469 (2019)
Article DOI: 10.1016/j.bmc.2018.11.020
BindingDB Entry DOI: 10.7270/Q2HD7ZZ6 |
More data for this
Ligand-Target Pair | |
Transcription initiation factor TFIID subunit 1-like
(Homo sapiens (Human)) | BDBM50504160

(CHEMBL3133807 | US11773085, Compound B25)Show SMILES CCOC(=O)Nc1cc(nn2c(C)nnc12)-c1ccc(C)c(NS(C)(=O)=O)c1 Show InChI InChI=1S/C17H20N6O4S/c1-5-27-17(24)18-15-9-14(21-23-11(3)19-20-16(15)23)12-7-6-10(2)13(8-12)22-28(4,25)26/h6-9,22H,5H2,1-4H3,(H,18,24) | PDB
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
PDB
UniChem
| PDB
PubMed
| n/a | n/a | n/a | 2.50E+4 | n/a | n/a | n/a | n/a | n/a |
University of Oxford
Curated by ChEMBL
| Assay Description
Reverse ITC (compound as receptor). Domain start/stop: M1401-D1522 |
Sci Adv 2: (2016)
BindingDB Entry DOI: 10.7270/Q24J0JPZ |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Transcription initiation factor TFIID subunit 1-like
(Homo sapiens (Human)) | BDBM50455480
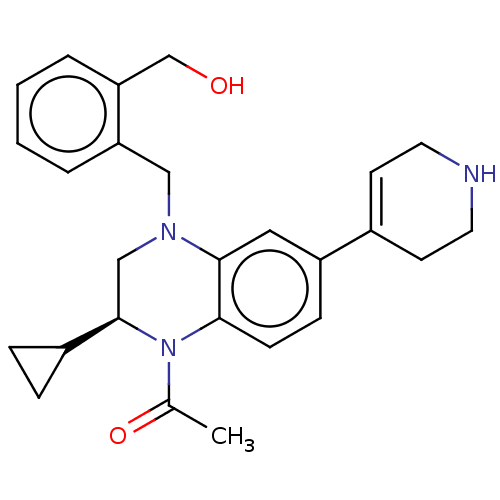
(CHEMBL4218735)Show SMILES CC(=O)N1[C@H](CN(Cc2ccccc2CO)c2cc(ccc12)C1=CCNCC1)C1CC1 |r,t:25| Show InChI InChI=1S/C26H31N3O2/c1-18(31)29-24-9-8-21(19-10-12-27-13-11-19)14-25(24)28(16-26(29)20-6-7-20)15-22-4-2-3-5-23(22)17-30/h2-5,8-10,14,20,26-27,30H,6-7,11-13,15-17H2,1H3/t26-/m1/s1 | PDB
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
PDB
UniChem
| Article
PubMed
| n/a | n/a | n/a | <3.02E+4 | n/a | n/a | n/a | n/a | n/a |
University of Strathclyde
Curated by ChEMBL
| Assay Description
Binding affinity to human partial length DNA-tagged TAF1L BD2 expressed in bacteria by BROMOscan method |
J Med Chem 61: 4317-4334 (2018)
Article DOI: 10.1021/acs.jmedchem.7b01666
BindingDB Entry DOI: 10.7270/Q27H1N6W |
More data for this
Ligand-Target Pair | |
Transcription initiation factor TFIID subunit 1-like
(Homo sapiens (Human)) | BDBM50269687
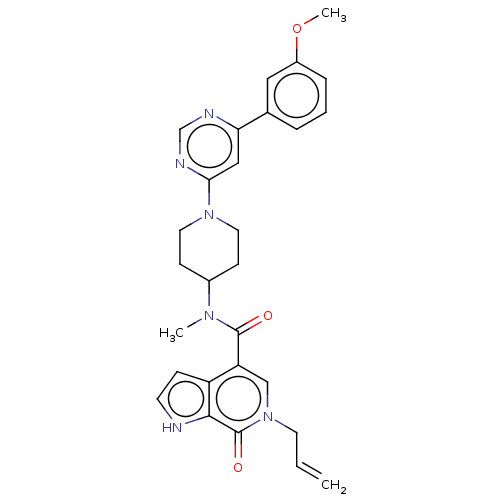
(CHEMBL4073178)Show SMILES COc1cccc(c1)-c1cc(ncn1)N1CCC(CC1)N(C)C(=O)c1cn(CC=C)c(=O)c2[nH]ccc12 Show InChI InChI=1S/C28H30N6O3/c1-4-12-34-17-23(22-8-11-29-26(22)28(34)36)27(35)32(2)20-9-13-33(14-10-20)25-16-24(30-18-31-25)19-6-5-7-21(15-19)37-3/h4-8,11,15-18,20,29H,1,9-10,12-14H2,2-3H3 | PDB
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | 2.40E+6 | n/a | n/a | n/a | n/a | n/a |
Genentech Inc., 1 DNA Way, South San Francisco, California 94080, United States.
Curated by ChEMBL
| Assay Description
Binding affinity to human DNA-tagged TAF1L bromodomain 2 (1523 to 1654 residues) expressed in bacterial expression system by BROMOscan assay |
ACS Med Chem Lett 8: 737-741 (2017)
Article DOI: 10.1021/acsmedchemlett.7b00132
BindingDB Entry DOI: 10.7270/Q2862JXN |
More data for this
Ligand-Target Pair | |
Transcription initiation factor TFIID subunit 1-like
(Homo sapiens (Human)) | BDBM50098311
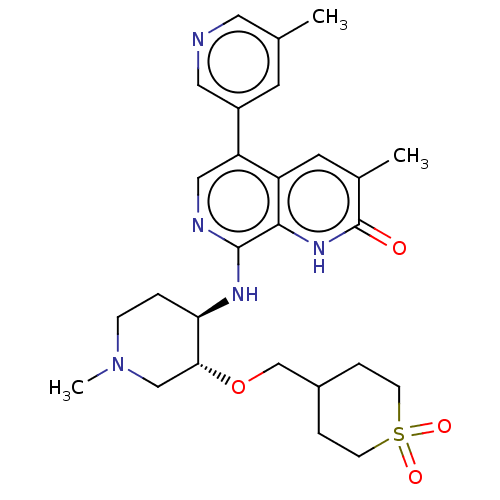
(CHEMBL3590408)Show SMILES CN1CC[C@@H](Nc2ncc(-c3cncc(C)c3)c3cc(C)c(=O)[nH]c23)[C@@H](C1)OCC1CCS(=O)(=O)CC1 |r| | PDB
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 126 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Binding affinity to TAF1L (unknown origin) by BROMOscan panel based assay |
J Med Chem 58: 6151-78 (2015)
Article DOI: 10.1021/acs.jmedchem.5b00773
BindingDB Entry DOI: 10.7270/Q2FN17Z1 |
More data for this
Ligand-Target Pair | |
Transcription initiation factor TFIID subunit 1-like
(Homo sapiens (Human)) | BDBM50260093
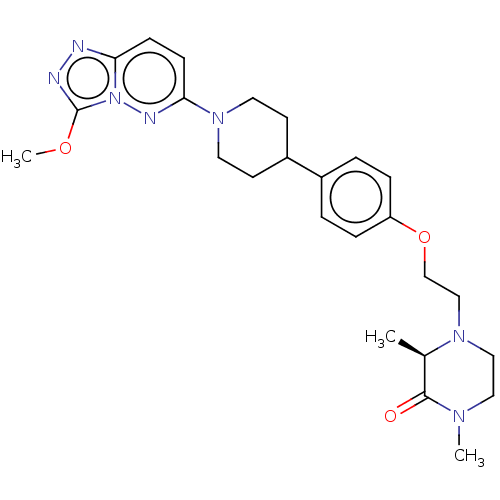
(CHEMBL4078100)Show SMILES COc1nnc2ccc(nn12)N1CCC(CC1)c1ccc(OCCN2CCN(C)C(=O)[C@H]2C)cc1 |r| Show InChI InChI=1S/C25H33N7O3/c1-18-24(33)29(2)14-15-30(18)16-17-35-21-6-4-19(5-7-21)20-10-12-31(13-11-20)23-9-8-22-26-27-25(34-3)32(22)28-23/h4-9,18,20H,10-17H2,1-3H3/t18-/m1/s1 | PDB
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
PDB
UniChem
Similars
| Article
PubMed
| 2.51E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Inhibition of human TAF1L bromodomain 2 expressed in bacterial expression system |
J Med Chem 59: 7801-17 (2016)
Article DOI: 10.1021/acs.jmedchem.6b00070
BindingDB Entry DOI: 10.7270/Q2697721 |
More data for this
Ligand-Target Pair | |
Transcription initiation factor TFIID subunit 1-like
(Homo sapiens (Human)) | BDBM50028142
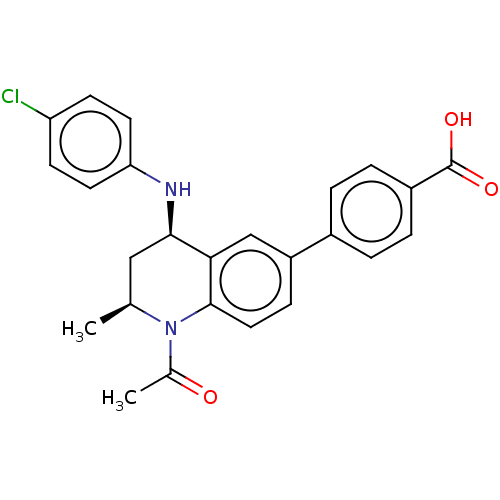
(CHEMBL2177300)Show SMILES C[C@H]1C[C@@H](Nc2ccc(Cl)cc2)c2cc(ccc2N1C(C)=O)-c1ccc(cc1)C(O)=O |r| Show InChI InChI=1S/C25H23ClN2O3/c1-15-13-23(27-21-10-8-20(26)9-11-21)22-14-19(7-12-24(22)28(15)16(2)29)17-3-5-18(6-4-17)25(30)31/h3-12,14-15,23,27H,13H2,1-2H3,(H,30,31)/t15-,23+/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
PDB
UniChem
Similars
| Article
PubMed
| n/a | n/a | 3.16E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline R&D
Curated by ChEMBL
| Assay Description
Inhibition of human TAF1L by BROMOscan assay |
J Med Chem 57: 8111-31 (2014)
Article DOI: 10.1021/jm5010539
BindingDB Entry DOI: 10.7270/Q23R0VGR |
More data for this
Ligand-Target Pair | |
Transcription initiation factor TFIID subunit 1-like
(Homo sapiens (Human)) | BDBM50591066
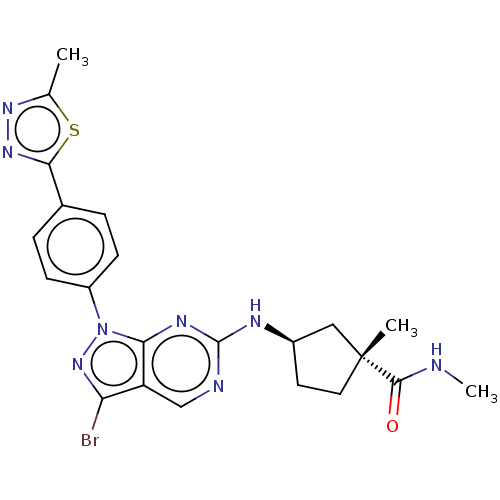
(CHEMBL5190023)Show SMILES CNC(=O)[C@]1(C)CC[C@H](C1)Nc1ncc2c(Br)nn(-c3ccc(cc3)-c3nnc(C)s3)c2n1 |r| | PDB
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 124 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c00736
BindingDB Entry DOI: 10.7270/Q27H1PJS |
More data for this
Ligand-Target Pair | |
Transcription initiation factor TFIID subunit 1-like
(Homo sapiens (Human)) | BDBM342597
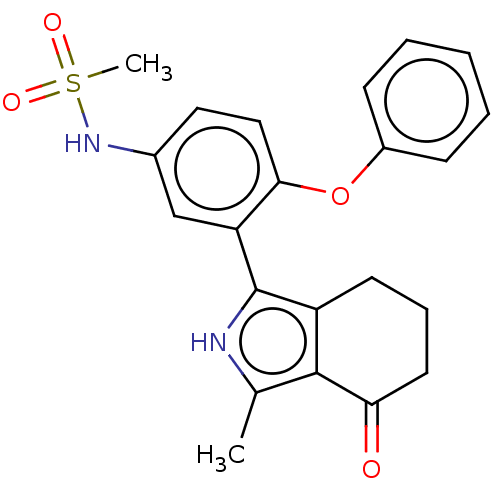
(N-[3-(3-methyl-4-oxo-4,5,6,7-tetrahydro-2H-isoindo...)Show SMILES Cc1[nH]c(c2CCCC(=O)c12)-c1cc(NS(C)(=O)=O)ccc1Oc1ccccc1 Show InChI InChI=1S/C22H22N2O4S/c1-14-21-17(9-6-10-19(21)25)22(23-14)18-13-15(24-29(2,26)27)11-12-20(18)28-16-7-4-3-5-8-16/h3-5,7-8,11-13,23-24H,6,9-10H2,1-2H3 | PDB
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
PDB
UniChem
| Article
PubMed
| n/a | n/a | >1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
AbbVie Inc., Oncology Discovery, 1 North Waukegan Rd., North Chicago, IL 60064, USA.
Curated by ChEMBL
| Assay Description
Inhibition of human TAF1L (Q1523 to D1654 residues) expressed in bacterial expression system by BROMOscan assay |
Bioorg Med Chem Lett 27: 2225-2233 (2017)
Article DOI: 10.1016/j.bmcl.2017.02.057
BindingDB Entry DOI: 10.7270/Q20P12FJ |
More data for this
Ligand-Target Pair | |
Transcription initiation factor TFIID subunit 1-like
(Homo sapiens (Human)) | BDBM50256397
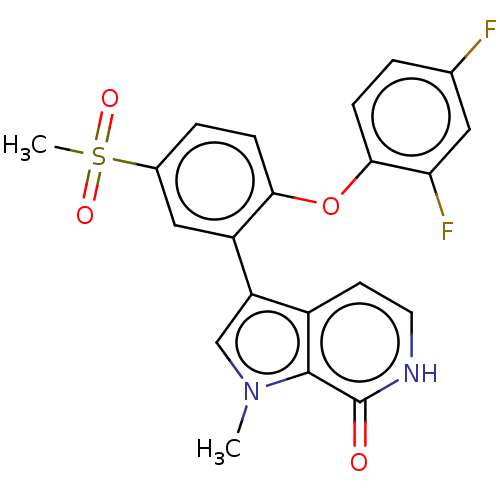
(CHEMBL4088466)Show SMILES Cn1cc(-c2cc(ccc2Oc2ccc(F)cc2F)S(C)(=O)=O)c2cc[nH]c(=O)c12 Show InChI InChI=1S/C21H16F2N2O4S/c1-25-11-16(14-7-8-24-21(26)20(14)25)15-10-13(30(2,27)28)4-6-18(15)29-19-5-3-12(22)9-17(19)23/h3-11H,1-2H3,(H,24,26) | PDB
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | >1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
AbbVie Inc., Oncology Discovery, 1 North Waukegan Rd., North Chicago, IL 60064, USA.
Curated by ChEMBL
| Assay Description
Inhibition of human TAF1L (Q1523 to D1654 residues) expressed in bacterial expression system by BROMOscan assay |
Bioorg Med Chem Lett 27: 2225-2233 (2017)
Article DOI: 10.1016/j.bmcl.2017.02.057
BindingDB Entry DOI: 10.7270/Q20P12FJ |
More data for this
Ligand-Target Pair | |
Transcription initiation factor TFIID subunit 1-like
(Homo sapiens (Human)) | BDBM50519662
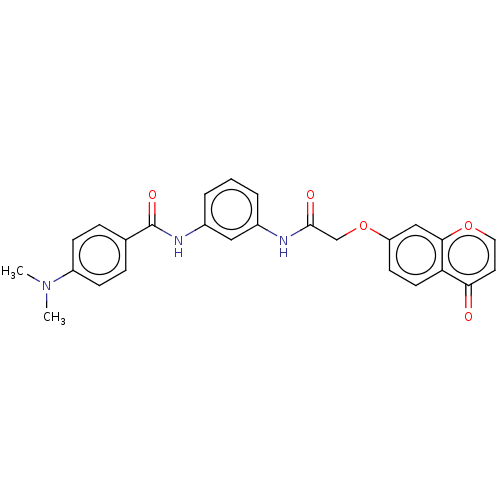
(CHEMBL4438748)Show SMILES CN(C)c1ccc(cc1)C(=O)Nc1cccc(NC(=O)COc2ccc3c(c2)occc3=O)c1 Show InChI InChI=1S/C26H23N3O5/c1-29(2)20-8-6-17(7-9-20)26(32)28-19-5-3-4-18(14-19)27-25(31)16-34-21-10-11-22-23(30)12-13-33-24(22)15-21/h3-15H,16H2,1-2H3,(H,27,31)(H,28,32) | PDB
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of recombinant human TAF1L (1428 to end residues) using RRRFRPASPLRGP as substrate incubated for 120 mins in presence of [gamma33P]ATP by ... |
J Med Chem 62: 10691-10710 (2019)
Article DOI: 10.1021/acs.jmedchem.9b01143
BindingDB Entry DOI: 10.7270/Q2MC93FG |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data