

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Hormone-sensitive lipase (Homo sapiens (Human)) | BDBM121019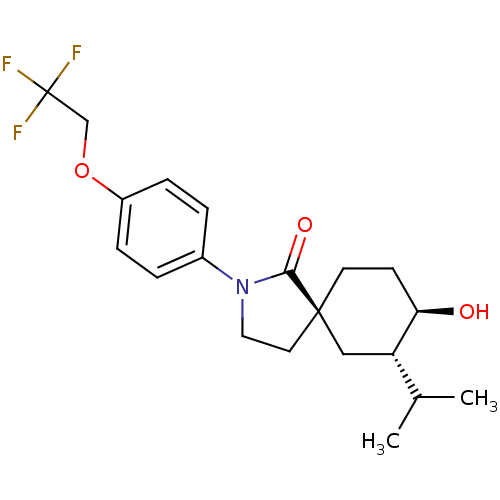 (US8722721, 11) | Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 2.40 | n/a | n/a | n/a | n/a | 7.2 | 4 |
Hoffmann-La Roche Inc. US Patent | Assay Description HSL enzyme activity was measured by a colorimetric assay using 2,3-dimercapto-1-propanol tributyrate (Aldrich, St. Louis, Mo.) as a substrate. Typica... | US Patent US8722721 (2014) BindingDB Entry DOI: 10.7270/Q2F76B7M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Hormone-sensitive lipase (Homo sapiens (Human)) | BDBM121018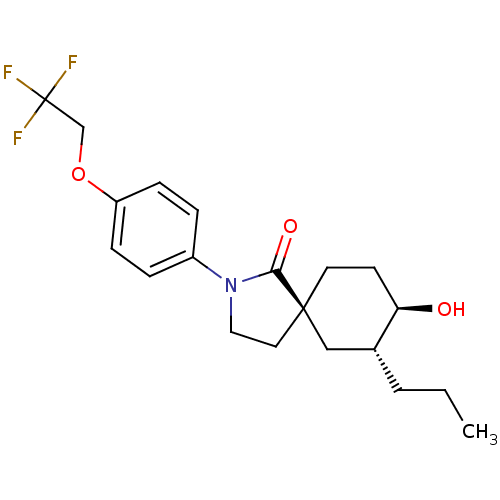 (US8722721, 10) | Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 3.70 | n/a | n/a | n/a | n/a | 7.2 | 4 |
Hoffmann-La Roche Inc. US Patent | Assay Description HSL enzyme activity was measured by a colorimetric assay using 2,3-dimercapto-1-propanol tributyrate (Aldrich, St. Louis, Mo.) as a substrate. Typica... | US Patent US8722721 (2014) BindingDB Entry DOI: 10.7270/Q2F76B7M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Hormone-sensitive lipase (Homo sapiens (Human)) | BDBM121011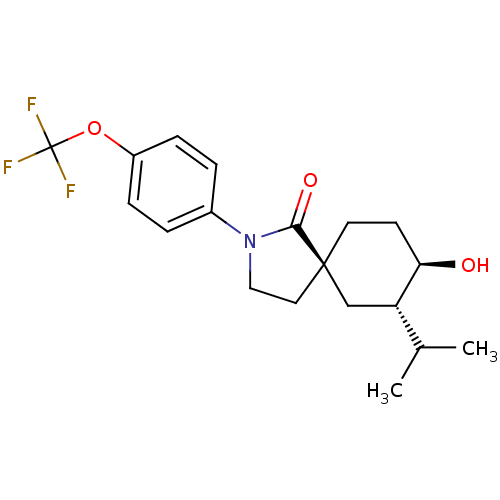 (US8722721, 2) | Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 16.2 | n/a | n/a | n/a | n/a | 7.2 | 4 |
Hoffmann-La Roche Inc. US Patent | Assay Description HSL enzyme activity was measured by a colorimetric assay using 2,3-dimercapto-1-propanol tributyrate (Aldrich, St. Louis, Mo.) as a substrate. Typica... | US Patent US8722721 (2014) BindingDB Entry DOI: 10.7270/Q2F76B7M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Hormone-sensitive lipase (Homo sapiens (Human)) | BDBM121027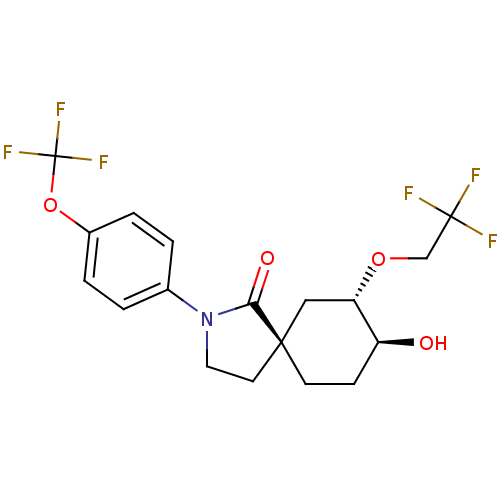 (US8722721, 7) | Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 42.1 | n/a | n/a | n/a | n/a | 7.2 | 4 |
Hoffmann-La Roche Inc. US Patent | Assay Description HSL enzyme activity was measured by a colorimetric assay using 2,3-dimercapto-1-propanol tributyrate (Aldrich, St. Louis, Mo.) as a substrate. Typica... | US Patent US8722721 (2014) BindingDB Entry DOI: 10.7270/Q2F76B7M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Hormone-sensitive lipase (Homo sapiens (Human)) | BDBM121014 (US8722721, 5 | US8722721, 6) | Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 66.4 | n/a | n/a | n/a | n/a | 7.2 | 4 |
Hoffmann-La Roche Inc. US Patent | Assay Description HSL enzyme activity was measured by a colorimetric assay using 2,3-dimercapto-1-propanol tributyrate (Aldrich, St. Louis, Mo.) as a substrate. Typica... | US Patent US8722721 (2014) BindingDB Entry DOI: 10.7270/Q2F76B7M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Hormone-sensitive lipase (Homo sapiens (Human)) | BDBM121010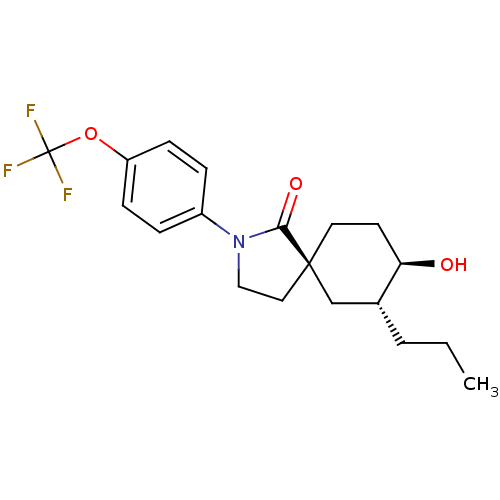 (US8722721, 1) | Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 67 | n/a | n/a | n/a | n/a | 7.2 | 4 |
Hoffmann-La Roche Inc. US Patent | Assay Description HSL enzyme activity was measured by a colorimetric assay using 2,3-dimercapto-1-propanol tributyrate (Aldrich, St. Louis, Mo.) as a substrate. Typica... | US Patent US8722721 (2014) BindingDB Entry DOI: 10.7270/Q2F76B7M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Hormone-sensitive lipase (Homo sapiens (Human)) | BDBM121017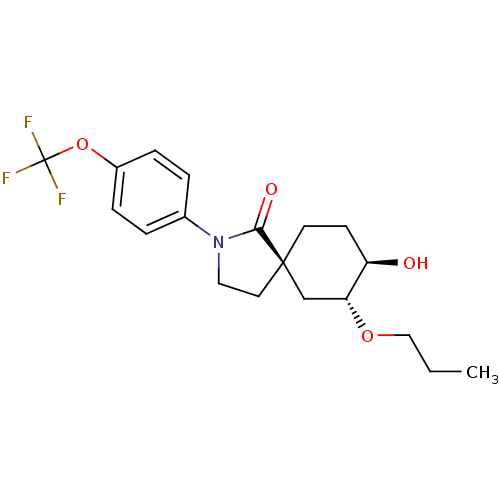 (US8722721, 9) | Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 73.3 | n/a | n/a | n/a | n/a | 7.2 | 4 |
Hoffmann-La Roche Inc. US Patent | Assay Description HSL enzyme activity was measured by a colorimetric assay using 2,3-dimercapto-1-propanol tributyrate (Aldrich, St. Louis, Mo.) as a substrate. Typica... | US Patent US8722721 (2014) BindingDB Entry DOI: 10.7270/Q2F76B7M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Hormone-sensitive lipase (Homo sapiens (Human)) | BDBM121023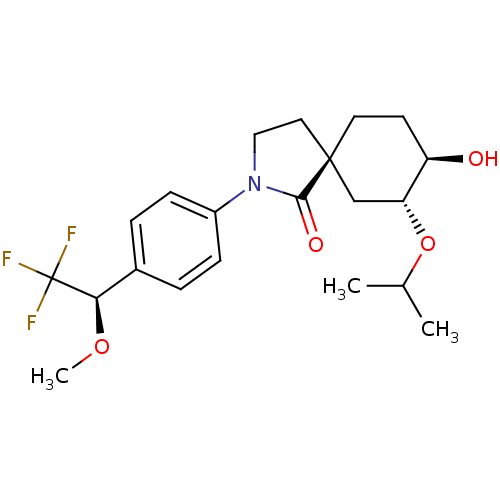 (US8722721, 15) | Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 136 | n/a | n/a | n/a | n/a | 7.2 | 4 |
Hoffmann-La Roche Inc. US Patent | Assay Description HSL enzyme activity was measured by a colorimetric assay using 2,3-dimercapto-1-propanol tributyrate (Aldrich, St. Louis, Mo.) as a substrate. Typica... | US Patent US8722721 (2014) BindingDB Entry DOI: 10.7270/Q2F76B7M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Hormone-sensitive lipase (Homo sapiens (Human)) | BDBM121014 (US8722721, 5 | US8722721, 6) | Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 158 | n/a | n/a | n/a | n/a | 7.2 | 4 |
Hoffmann-La Roche Inc. US Patent | Assay Description HSL enzyme activity was measured by a colorimetric assay using 2,3-dimercapto-1-propanol tributyrate (Aldrich, St. Louis, Mo.) as a substrate. Typica... | US Patent US8722721 (2014) BindingDB Entry DOI: 10.7270/Q2F76B7M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Hormone-sensitive lipase (Homo sapiens (Human)) | BDBM121024 (US8722721, 16) | Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 175 | n/a | n/a | n/a | n/a | 7.2 | 4 |
Hoffmann-La Roche Inc. US Patent | Assay Description HSL enzyme activity was measured by a colorimetric assay using 2,3-dimercapto-1-propanol tributyrate (Aldrich, St. Louis, Mo.) as a substrate. Typica... | US Patent US8722721 (2014) BindingDB Entry DOI: 10.7270/Q2F76B7M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Hormone-sensitive lipase (Homo sapiens (Human)) | BDBM121026 (US8722721, 18) | Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 185 | n/a | n/a | n/a | n/a | 7.2 | 4 |
Hoffmann-La Roche Inc. US Patent | Assay Description HSL enzyme activity was measured by a colorimetric assay using 2,3-dimercapto-1-propanol tributyrate (Aldrich, St. Louis, Mo.) as a substrate. Typica... | US Patent US8722721 (2014) BindingDB Entry DOI: 10.7270/Q2F76B7M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Hormone-sensitive lipase (Homo sapiens (Human)) | BDBM121012 (US8722721, 3) | Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 189 | n/a | n/a | n/a | n/a | 7.2 | 4 |
Hoffmann-La Roche Inc. US Patent | Assay Description HSL enzyme activity was measured by a colorimetric assay using 2,3-dimercapto-1-propanol tributyrate (Aldrich, St. Louis, Mo.) as a substrate. Typica... | US Patent US8722721 (2014) BindingDB Entry DOI: 10.7270/Q2F76B7M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Hormone-sensitive lipase (Homo sapiens (Human)) | BDBM121013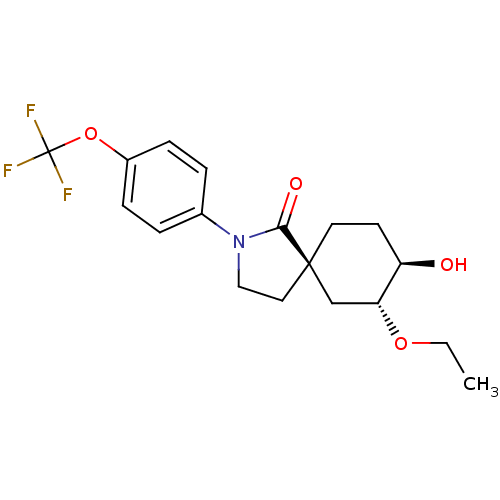 (US8722721, 4) | Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 198 | n/a | n/a | n/a | n/a | 7.2 | 4 |
Hoffmann-La Roche Inc. US Patent | Assay Description HSL enzyme activity was measured by a colorimetric assay using 2,3-dimercapto-1-propanol tributyrate (Aldrich, St. Louis, Mo.) as a substrate. Typica... | US Patent US8722721 (2014) BindingDB Entry DOI: 10.7270/Q2F76B7M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Hormone-sensitive lipase (Homo sapiens (Human)) | BDBM121016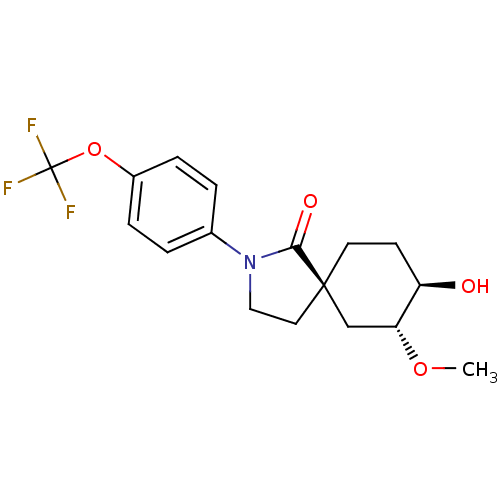 (US8722721, 8) | Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 343 | n/a | n/a | n/a | n/a | 7.2 | 4 |
Hoffmann-La Roche Inc. US Patent | Assay Description HSL enzyme activity was measured by a colorimetric assay using 2,3-dimercapto-1-propanol tributyrate (Aldrich, St. Louis, Mo.) as a substrate. Typica... | US Patent US8722721 (2014) BindingDB Entry DOI: 10.7270/Q2F76B7M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Hormone-sensitive lipase (Homo sapiens (Human)) | BDBM121020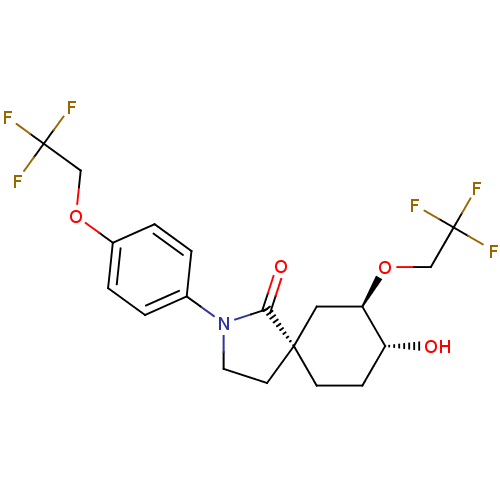 (US8722721, 12) | Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 350 | n/a | n/a | n/a | n/a | 7.2 | 4 |
Hoffmann-La Roche Inc. US Patent | Assay Description HSL enzyme activity was measured by a colorimetric assay using 2,3-dimercapto-1-propanol tributyrate (Aldrich, St. Louis, Mo.) as a substrate. Typica... | US Patent US8722721 (2014) BindingDB Entry DOI: 10.7270/Q2F76B7M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Hormone-sensitive lipase (Homo sapiens (Human)) | BDBM121022 (US8722721, 14) | Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 422 | n/a | n/a | n/a | n/a | 7.2 | 4 |
Hoffmann-La Roche Inc. US Patent | Assay Description HSL enzyme activity was measured by a colorimetric assay using 2,3-dimercapto-1-propanol tributyrate (Aldrich, St. Louis, Mo.) as a substrate. Typica... | US Patent US8722721 (2014) BindingDB Entry DOI: 10.7270/Q2F76B7M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Hormone-sensitive lipase (Homo sapiens (Human)) | BDBM121021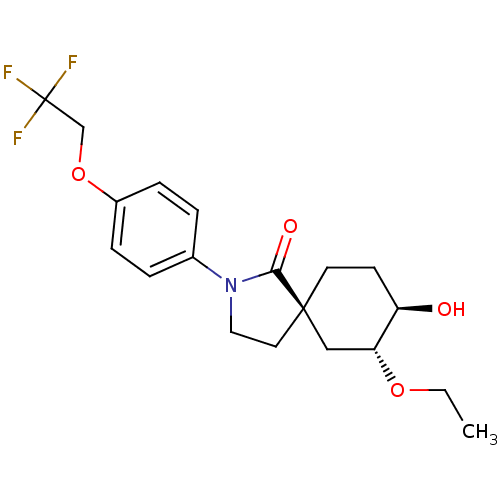 (US8722721, 13) | Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 994 | n/a | n/a | n/a | n/a | 7.2 | 4 |
Hoffmann-La Roche Inc. US Patent | Assay Description HSL enzyme activity was measured by a colorimetric assay using 2,3-dimercapto-1-propanol tributyrate (Aldrich, St. Louis, Mo.) as a substrate. Typica... | US Patent US8722721 (2014) BindingDB Entry DOI: 10.7270/Q2F76B7M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Hormone-sensitive lipase (Homo sapiens (Human)) | BDBM121025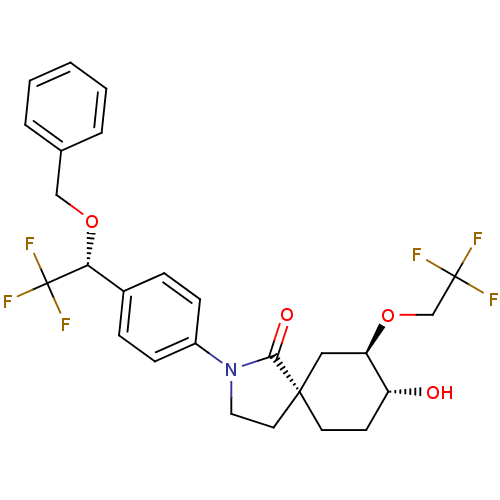 (US8722721, 17) | Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 1.07E+3 | n/a | n/a | n/a | n/a | 7.2 | 4 |
Hoffmann-La Roche Inc. US Patent | Assay Description HSL enzyme activity was measured by a colorimetric assay using 2,3-dimercapto-1-propanol tributyrate (Aldrich, St. Louis, Mo.) as a substrate. Typica... | US Patent US8722721 (2014) BindingDB Entry DOI: 10.7270/Q2F76B7M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||