

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Acetyl-CoA carboxylase 2 (Homo sapiens (Human)) | BDBM148657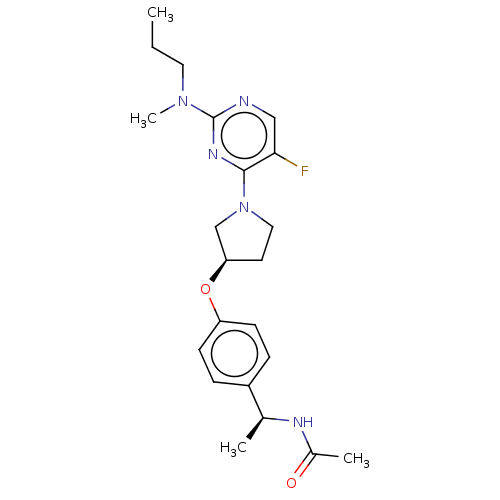 (US8962641, 8.13) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 30 | n/a | n/a | n/a | n/a | 7.5 | n/a |
Boehringer Ingelheim International GmbH US Patent | Assay Description Malonyl CoA formation by acetyl CoA carboxylases is stoichometrically linked to the consumption of ATP. ACC2 activity is measured in a NADH-linked ki... | US Patent US8962641 (2015) BindingDB Entry DOI: 10.7270/Q2H130Q5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetyl-CoA carboxylase 2 (Homo sapiens (Human)) | BDBM148629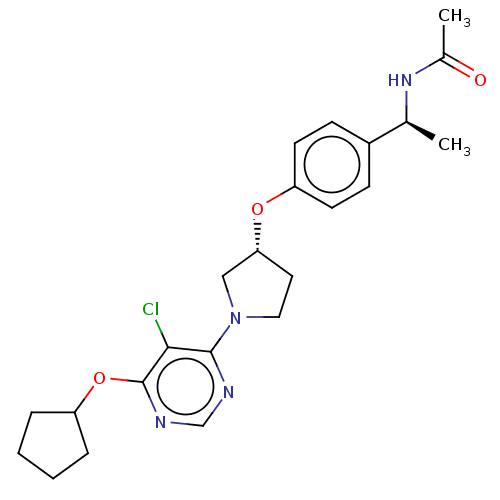 (US8962641, 7.53) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 32 | n/a | n/a | n/a | n/a | 7.5 | n/a |
Boehringer Ingelheim International GmbH US Patent | Assay Description Malonyl CoA formation by acetyl CoA carboxylases is stoichometrically linked to the consumption of ATP. ACC2 activity is measured in a NADH-linked ki... | US Patent US8962641 (2015) BindingDB Entry DOI: 10.7270/Q2H130Q5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetyl-CoA carboxylase 2 (Homo sapiens (Human)) | BDBM148653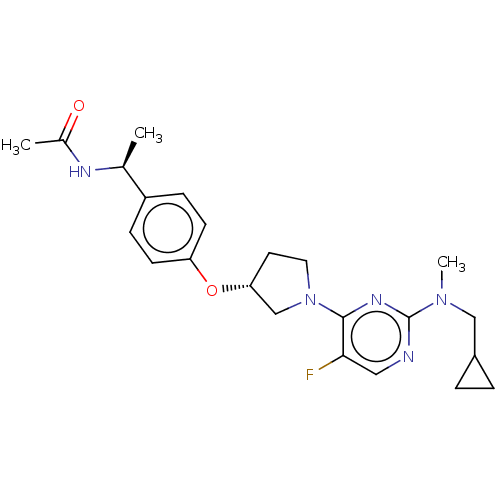 (US8962641, 8.9) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 33 | n/a | n/a | n/a | n/a | 7.5 | n/a |
Boehringer Ingelheim International GmbH US Patent | Assay Description Malonyl CoA formation by acetyl CoA carboxylases is stoichometrically linked to the consumption of ATP. ACC2 activity is measured in a NADH-linked ki... | US Patent US8962641 (2015) BindingDB Entry DOI: 10.7270/Q2H130Q5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetyl-CoA carboxylase 2 (Homo sapiens (Human)) | BDBM148576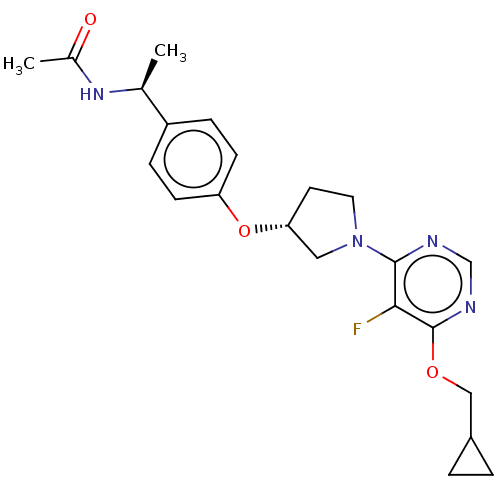 (US8962641, 7.1) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 35 | n/a | n/a | n/a | n/a | 7.5 | n/a |
Boehringer Ingelheim International GmbH US Patent | Assay Description Malonyl CoA formation by acetyl CoA carboxylases is stoichometrically linked to the consumption of ATP. ACC2 activity is measured in a NADH-linked ki... | US Patent US8962641 (2015) BindingDB Entry DOI: 10.7270/Q2H130Q5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetyl-CoA carboxylase 2 (Homo sapiens (Human)) | BDBM148603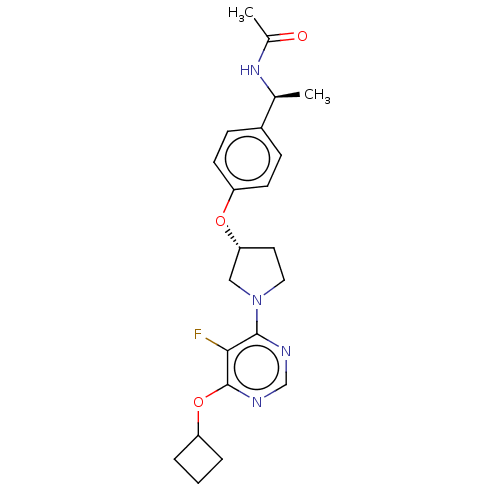 (US8962641, 7.27) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 35 | n/a | n/a | n/a | n/a | 7.5 | n/a |
Boehringer Ingelheim International GmbH US Patent | Assay Description Malonyl CoA formation by acetyl CoA carboxylases is stoichometrically linked to the consumption of ATP. ACC2 activity is measured in a NADH-linked ki... | US Patent US8962641 (2015) BindingDB Entry DOI: 10.7270/Q2H130Q5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetyl-CoA carboxylase 2 (Homo sapiens (Human)) | BDBM148596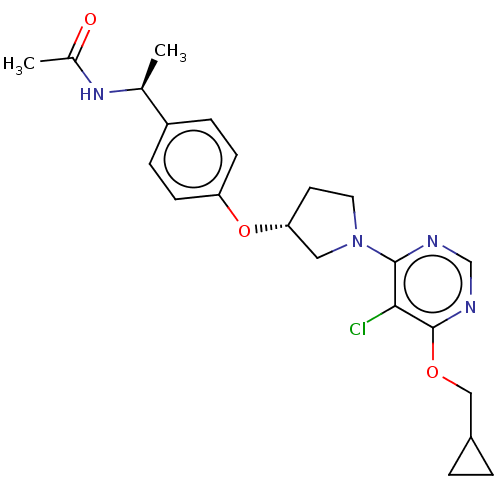 (US8962641, 7.20) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 35 | n/a | n/a | n/a | n/a | 7.5 | n/a |
Boehringer Ingelheim International GmbH US Patent | Assay Description Malonyl CoA formation by acetyl CoA carboxylases is stoichometrically linked to the consumption of ATP. ACC2 activity is measured in a NADH-linked ki... | US Patent US8962641 (2015) BindingDB Entry DOI: 10.7270/Q2H130Q5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetyl-CoA carboxylase 2 (Homo sapiens (Human)) | BDBM148722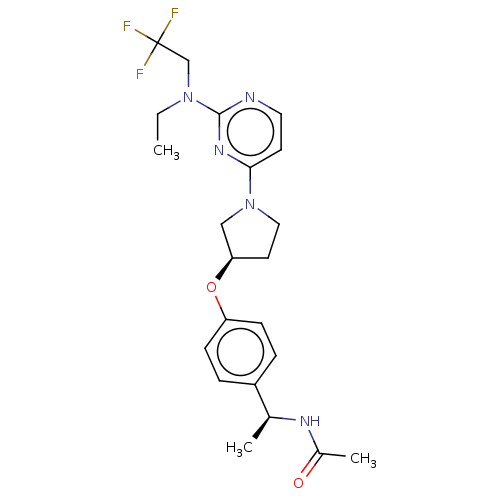 (US8962641, 8.79) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 37 | n/a | n/a | n/a | n/a | 7.5 | n/a |
Boehringer Ingelheim International GmbH US Patent | Assay Description Malonyl CoA formation by acetyl CoA carboxylases is stoichometrically linked to the consumption of ATP. ACC2 activity is measured in a NADH-linked ki... | US Patent US8962641 (2015) BindingDB Entry DOI: 10.7270/Q2H130Q5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetyl-CoA carboxylase 2 (Homo sapiens (Human)) | BDBM148638 (US8962641, 7.62) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 38 | n/a | n/a | n/a | n/a | 7.5 | n/a |
Boehringer Ingelheim International GmbH US Patent | Assay Description Malonyl CoA formation by acetyl CoA carboxylases is stoichometrically linked to the consumption of ATP. ACC2 activity is measured in a NADH-linked ki... | US Patent US8962641 (2015) BindingDB Entry DOI: 10.7270/Q2H130Q5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetyl-CoA carboxylase 2 (Homo sapiens (Human)) | BDBM148635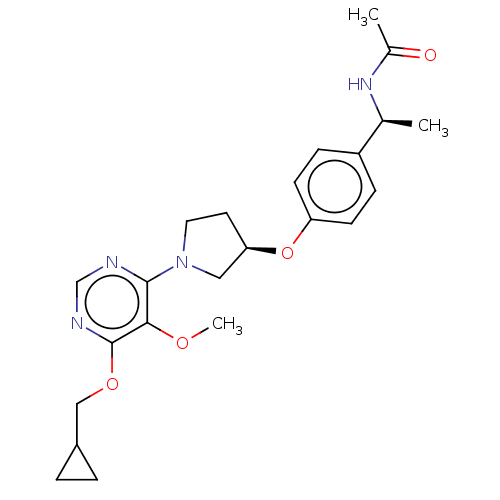 (US8962641, 7.59) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 39 | n/a | n/a | n/a | n/a | 7.5 | n/a |
Boehringer Ingelheim International GmbH US Patent | Assay Description Malonyl CoA formation by acetyl CoA carboxylases is stoichometrically linked to the consumption of ATP. ACC2 activity is measured in a NADH-linked ki... | US Patent US8962641 (2015) BindingDB Entry DOI: 10.7270/Q2H130Q5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetyl-CoA carboxylase 2 (Homo sapiens (Human)) | BDBM148502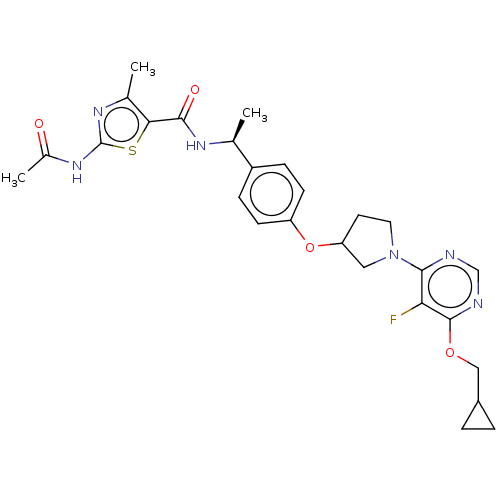 (US8962641, 2.19) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 39 | n/a | n/a | n/a | n/a | 7.5 | n/a |
Boehringer Ingelheim International GmbH US Patent | Assay Description Malonyl CoA formation by acetyl CoA carboxylases is stoichometrically linked to the consumption of ATP. ACC2 activity is measured in a NADH-linked ki... | US Patent US8962641 (2015) BindingDB Entry DOI: 10.7270/Q2H130Q5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetyl-CoA carboxylase 2 (Homo sapiens (Human)) | BDBM148617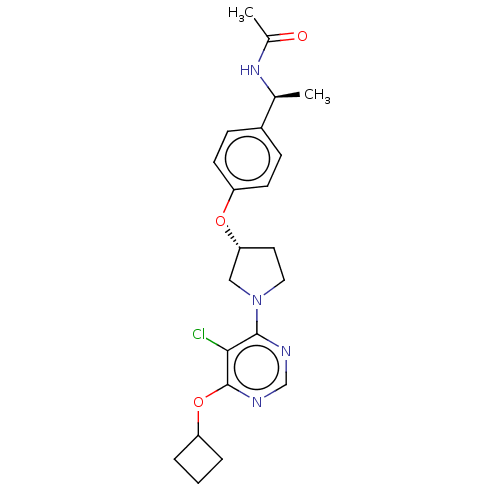 (US8962641, 7.41) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 39 | n/a | n/a | n/a | n/a | 7.5 | n/a |
Boehringer Ingelheim International GmbH US Patent | Assay Description Malonyl CoA formation by acetyl CoA carboxylases is stoichometrically linked to the consumption of ATP. ACC2 activity is measured in a NADH-linked ki... | US Patent US8962641 (2015) BindingDB Entry DOI: 10.7270/Q2H130Q5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetyl-CoA carboxylase 2 (Homo sapiens (Human)) | BDBM148517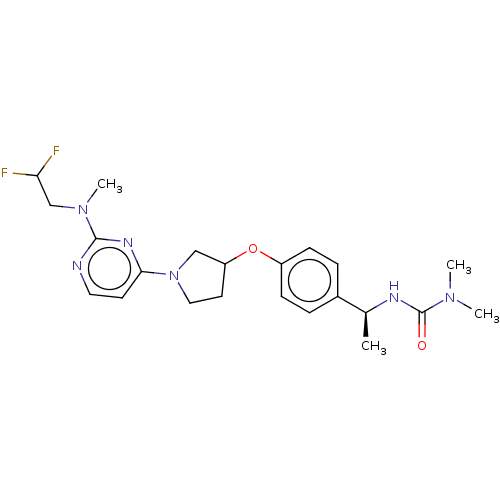 (US8962641, 3.1) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 40 | n/a | n/a | n/a | n/a | 7.5 | n/a |
Boehringer Ingelheim International GmbH US Patent | Assay Description Malonyl CoA formation by acetyl CoA carboxylases is stoichometrically linked to the consumption of ATP. ACC2 activity is measured in a NADH-linked ki... | US Patent US8962641 (2015) BindingDB Entry DOI: 10.7270/Q2H130Q5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetyl-CoA carboxylase 2 (Homo sapiens (Human)) | BDBM148756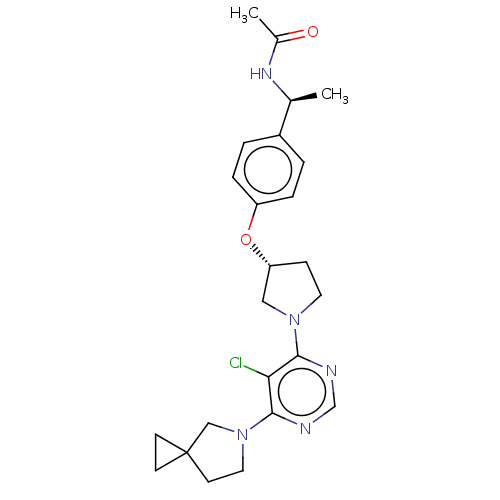 (US8962641, 8.113) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 40 | n/a | n/a | n/a | n/a | 7.5 | n/a |
Boehringer Ingelheim International GmbH US Patent | Assay Description Malonyl CoA formation by acetyl CoA carboxylases is stoichometrically linked to the consumption of ATP. ACC2 activity is measured in a NADH-linked ki... | US Patent US8962641 (2015) BindingDB Entry DOI: 10.7270/Q2H130Q5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetyl-CoA carboxylase 2 (Homo sapiens (Human)) | BDBM148833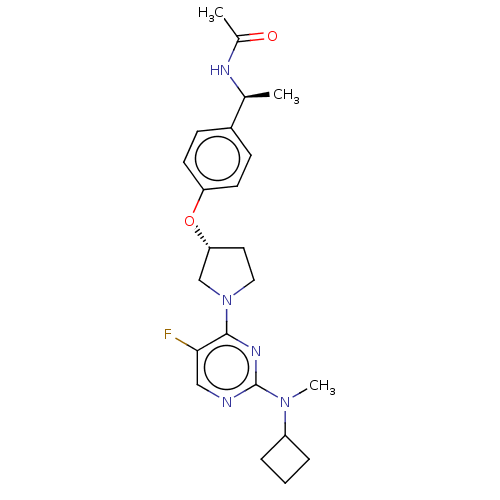 (US8962641, 8.190) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 41 | n/a | n/a | n/a | n/a | 7.5 | n/a |
Boehringer Ingelheim International GmbH US Patent | Assay Description Malonyl CoA formation by acetyl CoA carboxylases is stoichometrically linked to the consumption of ATP. ACC2 activity is measured in a NADH-linked ki... | US Patent US8962641 (2015) BindingDB Entry DOI: 10.7270/Q2H130Q5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetyl-CoA carboxylase 2 (Homo sapiens (Human)) | BDBM148952 (US8962641, 8.309) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 42 | n/a | n/a | n/a | n/a | 7.5 | n/a |
Boehringer Ingelheim International GmbH US Patent | Assay Description Malonyl CoA formation by acetyl CoA carboxylases is stoichometrically linked to the consumption of ATP. ACC2 activity is measured in a NADH-linked ki... | US Patent US8962641 (2015) BindingDB Entry DOI: 10.7270/Q2H130Q5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetyl-CoA carboxylase 2 (Homo sapiens (Human)) | BDBM148616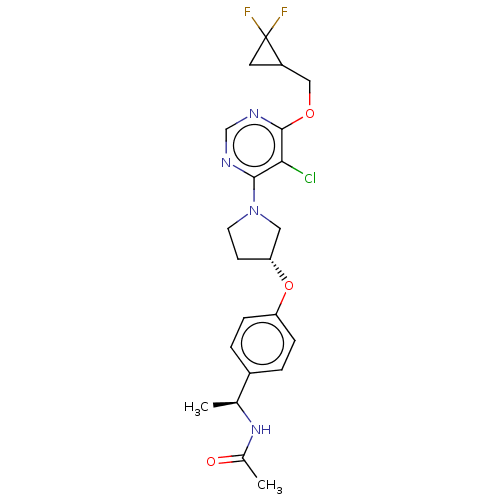 (US8962641, 7.40) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 42 | n/a | n/a | n/a | n/a | 7.5 | n/a |
Boehringer Ingelheim International GmbH US Patent | Assay Description Malonyl CoA formation by acetyl CoA carboxylases is stoichometrically linked to the consumption of ATP. ACC2 activity is measured in a NADH-linked ki... | US Patent US8962641 (2015) BindingDB Entry DOI: 10.7270/Q2H130Q5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetyl-CoA carboxylase 2 (Homo sapiens (Human)) | BDBM148652 (US8962641, 8.8) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 45 | n/a | n/a | n/a | n/a | 7.5 | n/a |
Boehringer Ingelheim International GmbH US Patent | Assay Description Malonyl CoA formation by acetyl CoA carboxylases is stoichometrically linked to the consumption of ATP. ACC2 activity is measured in a NADH-linked ki... | US Patent US8962641 (2015) BindingDB Entry DOI: 10.7270/Q2H130Q5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetyl-CoA carboxylase 2 (Homo sapiens (Human)) | BDBM148499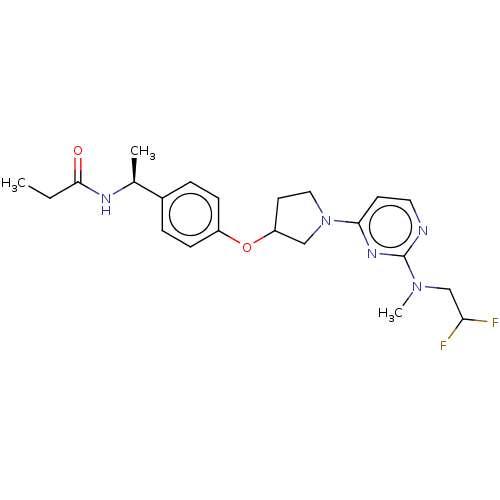 (US8962641, 2.16) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 45 | n/a | n/a | n/a | n/a | 7.5 | n/a |
Boehringer Ingelheim International GmbH US Patent | Assay Description Malonyl CoA formation by acetyl CoA carboxylases is stoichometrically linked to the consumption of ATP. ACC2 activity is measured in a NADH-linked ki... | US Patent US8962641 (2015) BindingDB Entry DOI: 10.7270/Q2H130Q5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetyl-CoA carboxylase 2 (Homo sapiens (Human)) | BDBM148597 (US8962641, 7.21) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 45 | n/a | n/a | n/a | n/a | 7.5 | n/a |
Boehringer Ingelheim International GmbH US Patent | Assay Description Malonyl CoA formation by acetyl CoA carboxylases is stoichometrically linked to the consumption of ATP. ACC2 activity is measured in a NADH-linked ki... | US Patent US8962641 (2015) BindingDB Entry DOI: 10.7270/Q2H130Q5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetyl-CoA carboxylase 2 (Homo sapiens (Human)) | BDBM148527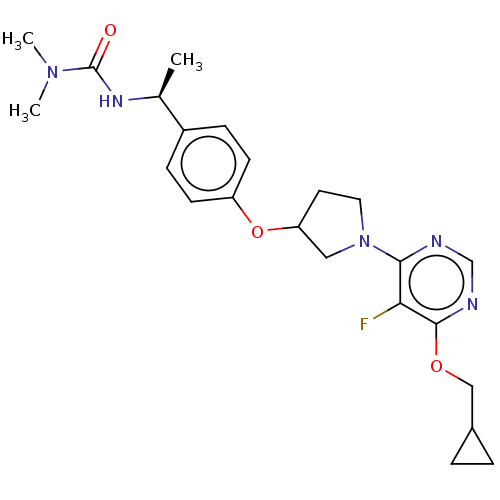 (US8962641, 3.10) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 45 | n/a | n/a | n/a | n/a | 7.5 | n/a |
Boehringer Ingelheim International GmbH US Patent | Assay Description Malonyl CoA formation by acetyl CoA carboxylases is stoichometrically linked to the consumption of ATP. ACC2 activity is measured in a NADH-linked ki... | US Patent US8962641 (2015) BindingDB Entry DOI: 10.7270/Q2H130Q5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetyl-CoA carboxylase 2 (Homo sapiens (Human)) | BDBM148878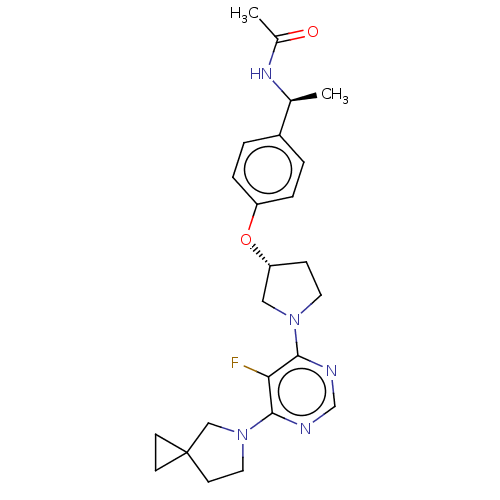 (US8962641, 8.235) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 45 | n/a | n/a | n/a | n/a | 7.5 | n/a |
Boehringer Ingelheim International GmbH US Patent | Assay Description Malonyl CoA formation by acetyl CoA carboxylases is stoichometrically linked to the consumption of ATP. ACC2 activity is measured in a NADH-linked ki... | US Patent US8962641 (2015) BindingDB Entry DOI: 10.7270/Q2H130Q5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetyl-CoA carboxylase 2 (Homo sapiens (Human)) | BDBM148505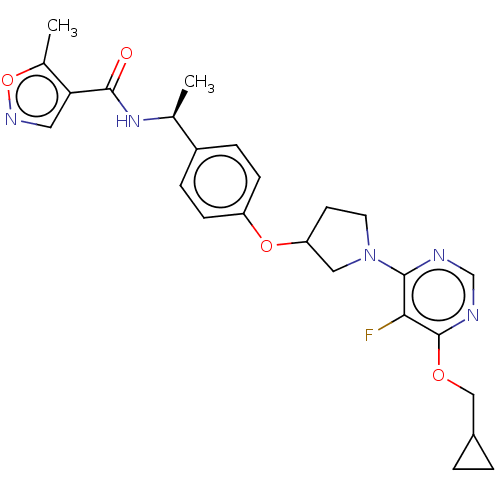 (US8962641, 2.22) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 48 | n/a | n/a | n/a | n/a | 7.5 | n/a |
Boehringer Ingelheim International GmbH US Patent | Assay Description Malonyl CoA formation by acetyl CoA carboxylases is stoichometrically linked to the consumption of ATP. ACC2 activity is measured in a NADH-linked ki... | US Patent US8962641 (2015) BindingDB Entry DOI: 10.7270/Q2H130Q5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetyl-CoA carboxylase 2 (Homo sapiens (Human)) | BDBM148607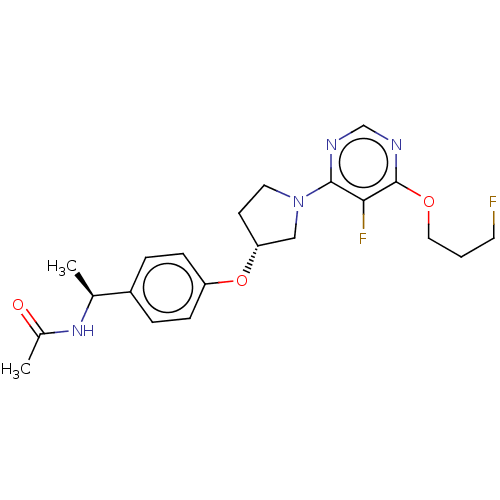 (US8962641, 7.31) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 49 | n/a | n/a | n/a | n/a | 7.5 | n/a |
Boehringer Ingelheim International GmbH US Patent | Assay Description Malonyl CoA formation by acetyl CoA carboxylases is stoichometrically linked to the consumption of ATP. ACC2 activity is measured in a NADH-linked ki... | US Patent US8962641 (2015) BindingDB Entry DOI: 10.7270/Q2H130Q5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetyl-CoA carboxylase 2 (Homo sapiens (Human)) | BDBM148547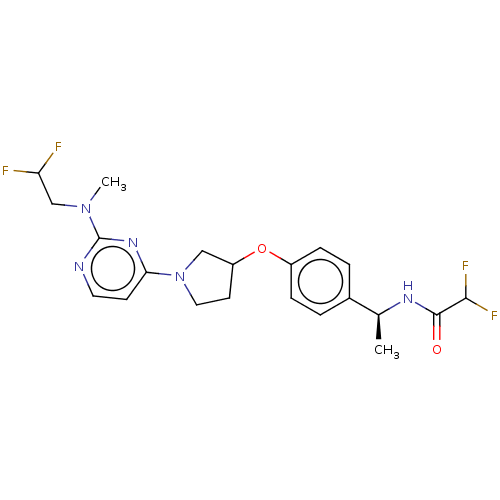 (US8962641, 5.2) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 49 | n/a | n/a | n/a | n/a | 7.5 | n/a |
Boehringer Ingelheim International GmbH US Patent | Assay Description Malonyl CoA formation by acetyl CoA carboxylases is stoichometrically linked to the consumption of ATP. ACC2 activity is measured in a NADH-linked ki... | US Patent US8962641 (2015) BindingDB Entry DOI: 10.7270/Q2H130Q5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetyl-CoA carboxylase 2 (Homo sapiens (Human)) | BDBM148782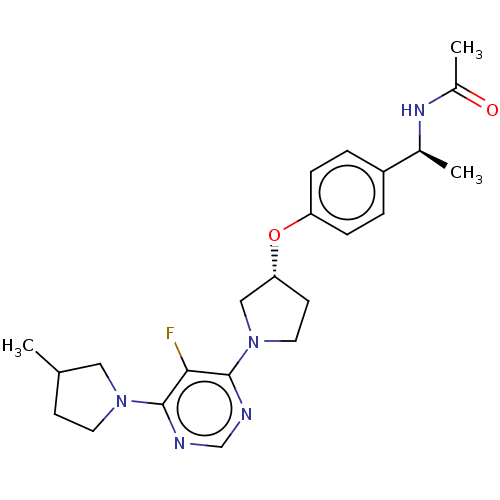 (US8962641, 8.139) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 49 | n/a | n/a | n/a | n/a | 7.5 | n/a |
Boehringer Ingelheim International GmbH US Patent | Assay Description Malonyl CoA formation by acetyl CoA carboxylases is stoichometrically linked to the consumption of ATP. ACC2 activity is measured in a NADH-linked ki... | US Patent US8962641 (2015) BindingDB Entry DOI: 10.7270/Q2H130Q5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetyl-CoA carboxylase 2 (Homo sapiens (Human)) | BDBM148711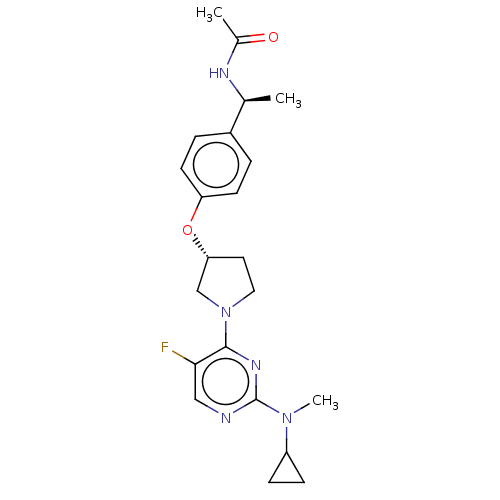 (US8962641, 8.68) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 50 | n/a | n/a | n/a | n/a | 7.5 | n/a |
Boehringer Ingelheim International GmbH US Patent | Assay Description Malonyl CoA formation by acetyl CoA carboxylases is stoichometrically linked to the consumption of ATP. ACC2 activity is measured in a NADH-linked ki... | US Patent US8962641 (2015) BindingDB Entry DOI: 10.7270/Q2H130Q5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetyl-CoA carboxylase 2 (Homo sapiens (Human)) | BDBM148530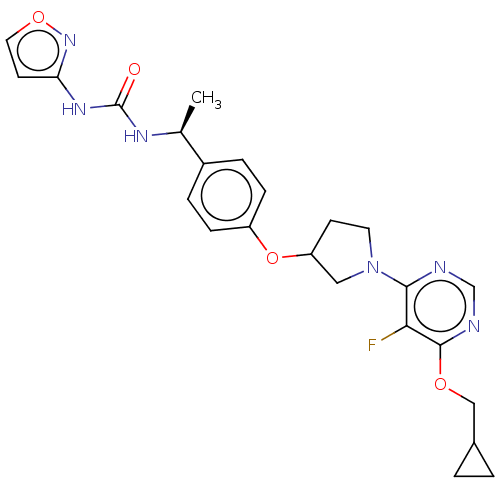 (US8962641, 3.13) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 50 | n/a | n/a | n/a | n/a | 7.5 | n/a |
Boehringer Ingelheim International GmbH US Patent | Assay Description Malonyl CoA formation by acetyl CoA carboxylases is stoichometrically linked to the consumption of ATP. ACC2 activity is measured in a NADH-linked ki... | US Patent US8962641 (2015) BindingDB Entry DOI: 10.7270/Q2H130Q5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetyl-CoA carboxylase 2 (Homo sapiens (Human)) | BDBM148936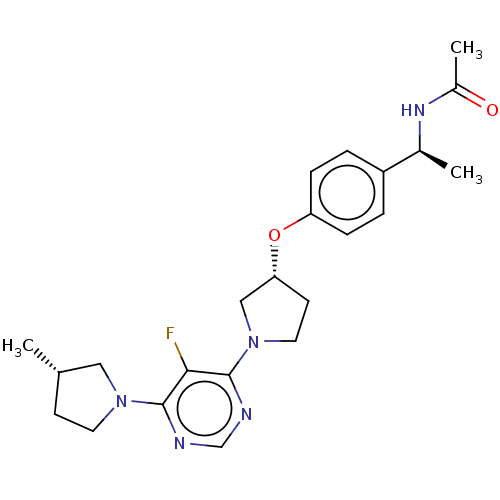 (US8962641, 8.293) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 50 | n/a | n/a | n/a | n/a | 7.5 | n/a |
Boehringer Ingelheim International GmbH US Patent | Assay Description Malonyl CoA formation by acetyl CoA carboxylases is stoichometrically linked to the consumption of ATP. ACC2 activity is measured in a NADH-linked ki... | US Patent US8962641 (2015) BindingDB Entry DOI: 10.7270/Q2H130Q5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetyl-CoA carboxylase 2 (Homo sapiens (Human)) | BDBM148869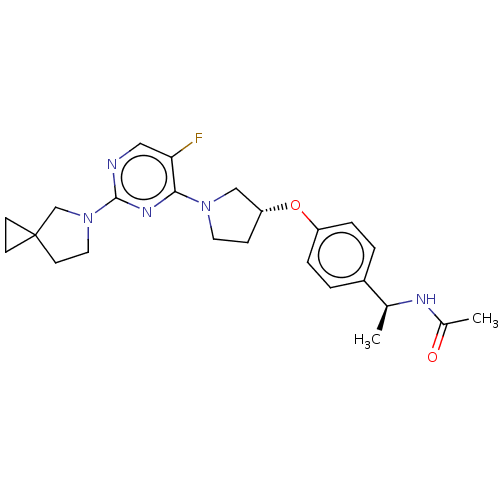 (US8962641, 8.226) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 52 | n/a | n/a | n/a | n/a | 7.5 | n/a |
Boehringer Ingelheim International GmbH US Patent | Assay Description Malonyl CoA formation by acetyl CoA carboxylases is stoichometrically linked to the consumption of ATP. ACC2 activity is measured in a NADH-linked ki... | US Patent US8962641 (2015) BindingDB Entry DOI: 10.7270/Q2H130Q5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetyl-CoA carboxylase 2 (Homo sapiens (Human)) | BDBM148953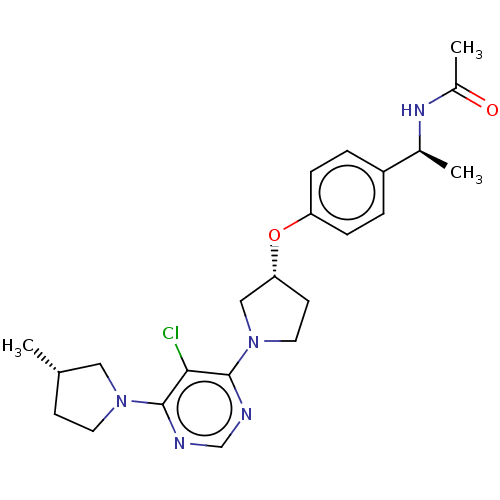 (US8962641, 8.310) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 52 | n/a | n/a | n/a | n/a | 7.5 | n/a |
Boehringer Ingelheim International GmbH US Patent | Assay Description Malonyl CoA formation by acetyl CoA carboxylases is stoichometrically linked to the consumption of ATP. ACC2 activity is measured in a NADH-linked ki... | US Patent US8962641 (2015) BindingDB Entry DOI: 10.7270/Q2H130Q5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetyl-CoA carboxylase 2 (Homo sapiens (Human)) | BDBM148937 (US8962641, 8.294) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 52 | n/a | n/a | n/a | n/a | 7.5 | n/a |
Boehringer Ingelheim International GmbH US Patent | Assay Description Malonyl CoA formation by acetyl CoA carboxylases is stoichometrically linked to the consumption of ATP. ACC2 activity is measured in a NADH-linked ki... | US Patent US8962641 (2015) BindingDB Entry DOI: 10.7270/Q2H130Q5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetyl-CoA carboxylase 2 (Homo sapiens (Human)) | BDBM148755 (US8962641, 8.112) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 53 | n/a | n/a | n/a | n/a | 7.5 | n/a |
Boehringer Ingelheim International GmbH US Patent | Assay Description Malonyl CoA formation by acetyl CoA carboxylases is stoichometrically linked to the consumption of ATP. ACC2 activity is measured in a NADH-linked ki... | US Patent US8962641 (2015) BindingDB Entry DOI: 10.7270/Q2H130Q5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetyl-CoA carboxylase 2 (Homo sapiens (Human)) | BDBM148503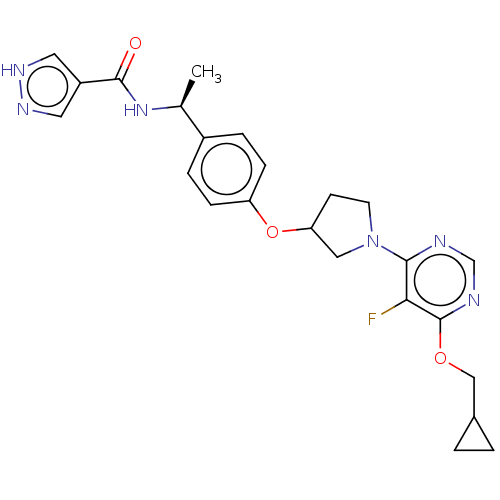 (US8962641, 2.20) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 53 | n/a | n/a | n/a | n/a | 7.5 | n/a |
Boehringer Ingelheim International GmbH US Patent | Assay Description Malonyl CoA formation by acetyl CoA carboxylases is stoichometrically linked to the consumption of ATP. ACC2 activity is measured in a NADH-linked ki... | US Patent US8962641 (2015) BindingDB Entry DOI: 10.7270/Q2H130Q5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetyl-CoA carboxylase 2 (Homo sapiens (Human)) | BDBM47172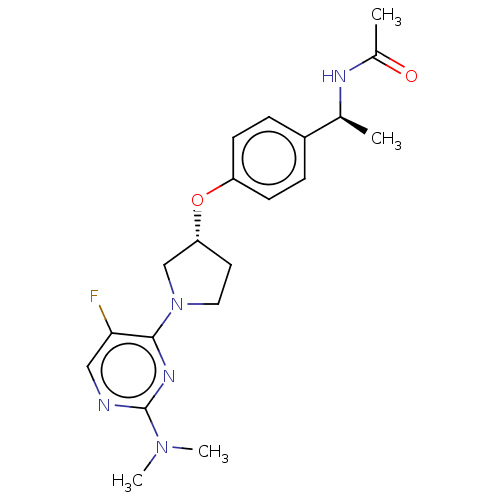 (US8962641, 8.1 | US8962641, 8.65) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 55 | n/a | n/a | n/a | n/a | 7.5 | n/a |
Boehringer Ingelheim International GmbH US Patent | Assay Description Malonyl CoA formation by acetyl CoA carboxylases is stoichometrically linked to the consumption of ATP. ACC2 activity is measured in a NADH-linked ki... | US Patent US8962641 (2015) BindingDB Entry DOI: 10.7270/Q2H130Q5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetyl-CoA carboxylase 2 (Homo sapiens (Human)) | BDBM148688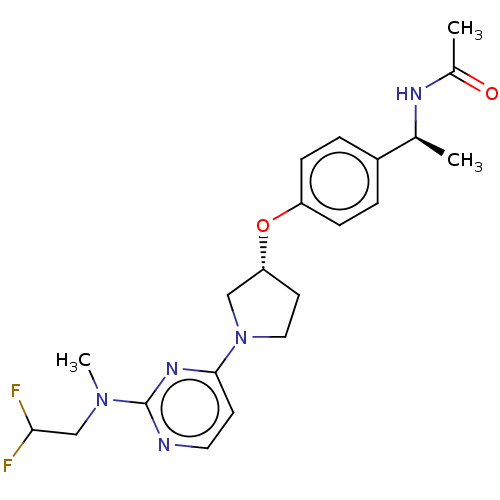 (US8962641, 8.44) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 55 | n/a | n/a | n/a | n/a | 7.5 | n/a |
Boehringer Ingelheim International GmbH US Patent | Assay Description Malonyl CoA formation by acetyl CoA carboxylases is stoichometrically linked to the consumption of ATP. ACC2 activity is measured in a NADH-linked ki... | US Patent US8962641 (2015) BindingDB Entry DOI: 10.7270/Q2H130Q5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetyl-CoA carboxylase 2 (Homo sapiens (Human)) | BDBM148801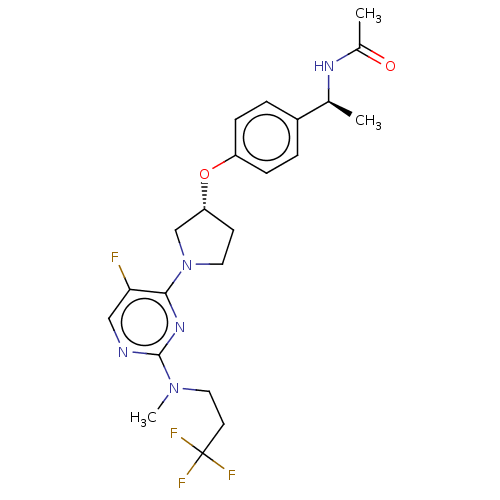 (US8962641, 8.158) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 56 | n/a | n/a | n/a | n/a | 7.5 | n/a |
Boehringer Ingelheim International GmbH US Patent | Assay Description Malonyl CoA formation by acetyl CoA carboxylases is stoichometrically linked to the consumption of ATP. ACC2 activity is measured in a NADH-linked ki... | US Patent US8962641 (2015) BindingDB Entry DOI: 10.7270/Q2H130Q5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetyl-CoA carboxylase 2 (Homo sapiens (Human)) | BDBM148926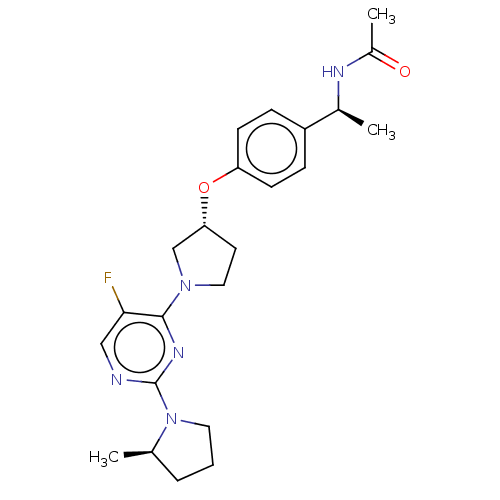 (US8962641, 8.283) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 57 | n/a | n/a | n/a | n/a | 7.5 | n/a |
Boehringer Ingelheim International GmbH US Patent | Assay Description Malonyl CoA formation by acetyl CoA carboxylases is stoichometrically linked to the consumption of ATP. ACC2 activity is measured in a NADH-linked ki... | US Patent US8962641 (2015) BindingDB Entry DOI: 10.7270/Q2H130Q5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetyl-CoA carboxylase 2 (Homo sapiens (Human)) | BDBM148655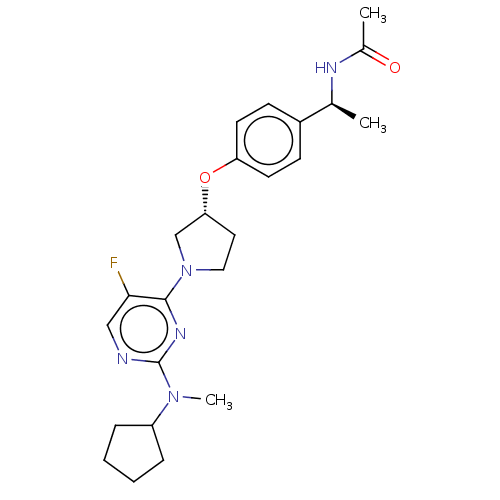 (US8962641, 8.11) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 58 | n/a | n/a | n/a | n/a | 7.5 | n/a |
Boehringer Ingelheim International GmbH US Patent | Assay Description Malonyl CoA formation by acetyl CoA carboxylases is stoichometrically linked to the consumption of ATP. ACC2 activity is measured in a NADH-linked ki... | US Patent US8962641 (2015) BindingDB Entry DOI: 10.7270/Q2H130Q5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetyl-CoA carboxylase 2 (Homo sapiens (Human)) | BDBM148623 (US8962641, 7.47) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 58 | n/a | n/a | n/a | n/a | 7.5 | n/a |
Boehringer Ingelheim International GmbH US Patent | Assay Description Malonyl CoA formation by acetyl CoA carboxylases is stoichometrically linked to the consumption of ATP. ACC2 activity is measured in a NADH-linked ki... | US Patent US8962641 (2015) BindingDB Entry DOI: 10.7270/Q2H130Q5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetyl-CoA carboxylase 2 (Homo sapiens (Human)) | BDBM148769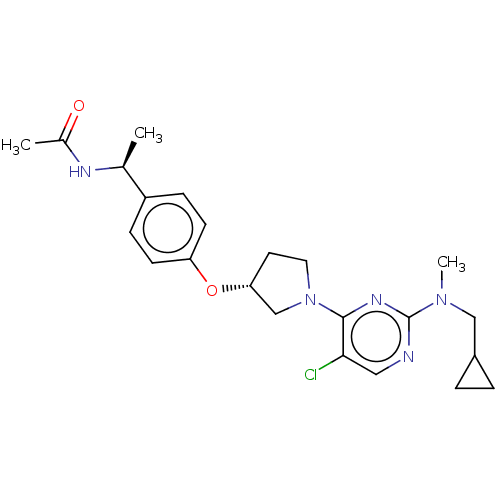 (US8962641, 8.126) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 58 | n/a | n/a | n/a | n/a | 7.5 | n/a |
Boehringer Ingelheim International GmbH US Patent | Assay Description Malonyl CoA formation by acetyl CoA carboxylases is stoichometrically linked to the consumption of ATP. ACC2 activity is measured in a NADH-linked ki... | US Patent US8962641 (2015) BindingDB Entry DOI: 10.7270/Q2H130Q5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetyl-CoA carboxylase 2 (Homo sapiens (Human)) | BDBM148704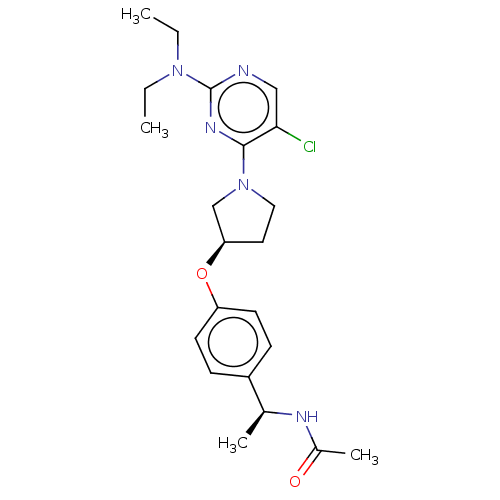 (US8962641, 8.60) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 59 | n/a | n/a | n/a | n/a | 7.5 | n/a |
Boehringer Ingelheim International GmbH US Patent | Assay Description Malonyl CoA formation by acetyl CoA carboxylases is stoichometrically linked to the consumption of ATP. ACC2 activity is measured in a NADH-linked ki... | US Patent US8962641 (2015) BindingDB Entry DOI: 10.7270/Q2H130Q5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetyl-CoA carboxylase 2 (Homo sapiens (Human)) | BDBM148713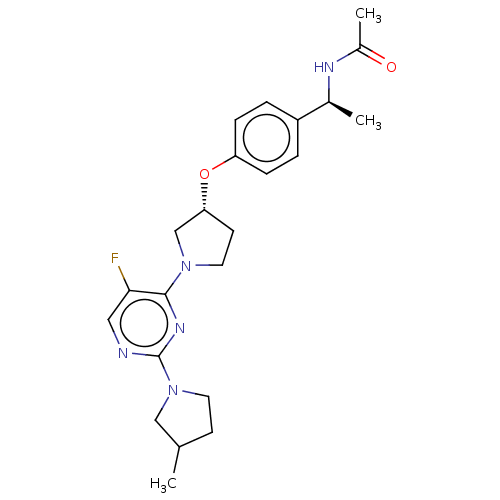 (US8962641, 8.70 | US8962641, 8.82) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 59 | n/a | n/a | n/a | n/a | 7.5 | n/a |
Boehringer Ingelheim International GmbH US Patent | Assay Description Malonyl CoA formation by acetyl CoA carboxylases is stoichometrically linked to the consumption of ATP. ACC2 activity is measured in a NADH-linked ki... | US Patent US8962641 (2015) BindingDB Entry DOI: 10.7270/Q2H130Q5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetyl-CoA carboxylase 2 (Homo sapiens (Human)) | BDBM148747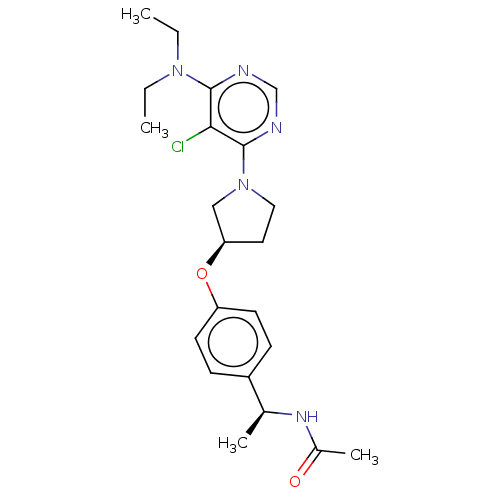 (US8962641, 8.104) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 59 | n/a | n/a | n/a | n/a | 7.5 | n/a |
Boehringer Ingelheim International GmbH US Patent | Assay Description Malonyl CoA formation by acetyl CoA carboxylases is stoichometrically linked to the consumption of ATP. ACC2 activity is measured in a NADH-linked ki... | US Patent US8962641 (2015) BindingDB Entry DOI: 10.7270/Q2H130Q5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetyl-CoA carboxylase 2 (Homo sapiens (Human)) | BDBM148545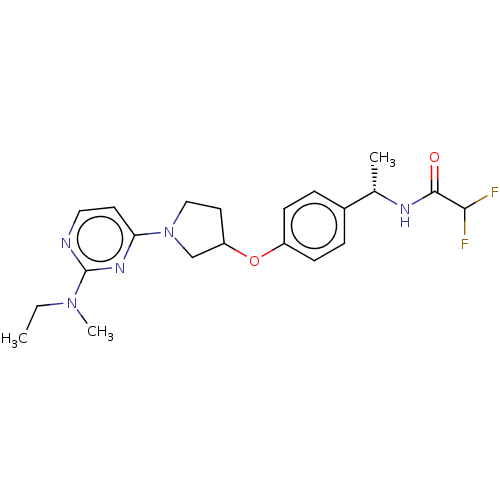 (US8962641, 5.1) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 59 | n/a | n/a | n/a | n/a | 7.5 | n/a |
Boehringer Ingelheim International GmbH US Patent | Assay Description Malonyl CoA formation by acetyl CoA carboxylases is stoichometrically linked to the consumption of ATP. ACC2 activity is measured in a NADH-linked ki... | US Patent US8962641 (2015) BindingDB Entry DOI: 10.7270/Q2H130Q5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetyl-CoA carboxylase 2 (Homo sapiens (Human)) | BDBM148897 (US8962641, 8.254) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 60 | n/a | n/a | n/a | n/a | 7.5 | n/a |
Boehringer Ingelheim International GmbH US Patent | Assay Description Malonyl CoA formation by acetyl CoA carboxylases is stoichometrically linked to the consumption of ATP. ACC2 activity is measured in a NADH-linked ki... | US Patent US8962641 (2015) BindingDB Entry DOI: 10.7270/Q2H130Q5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetyl-CoA carboxylase 2 (Homo sapiens (Human)) | BDBM148875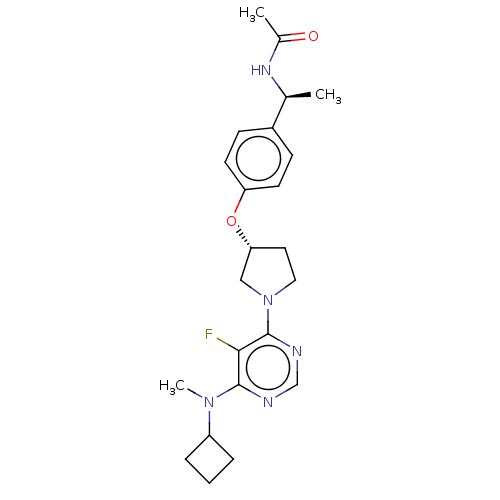 (US8962641, 8.232) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 60 | n/a | n/a | n/a | n/a | 7.5 | n/a |
Boehringer Ingelheim International GmbH US Patent | Assay Description Malonyl CoA formation by acetyl CoA carboxylases is stoichometrically linked to the consumption of ATP. ACC2 activity is measured in a NADH-linked ki... | US Patent US8962641 (2015) BindingDB Entry DOI: 10.7270/Q2H130Q5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetyl-CoA carboxylase 2 (Homo sapiens (Human)) | BDBM148615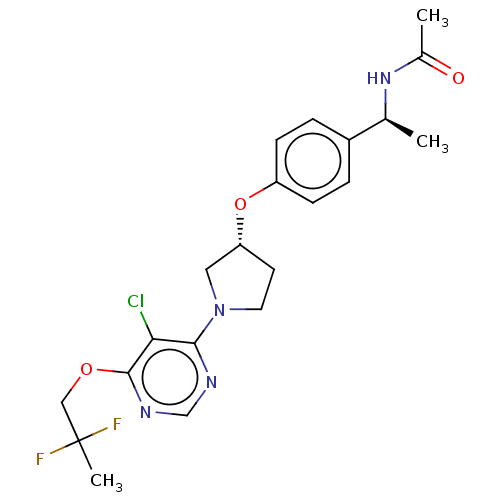 (US8962641, 7.39) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 60 | n/a | n/a | n/a | n/a | 7.5 | n/a |
Boehringer Ingelheim International GmbH US Patent | Assay Description Malonyl CoA formation by acetyl CoA carboxylases is stoichometrically linked to the consumption of ATP. ACC2 activity is measured in a NADH-linked ki... | US Patent US8962641 (2015) BindingDB Entry DOI: 10.7270/Q2H130Q5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetyl-CoA carboxylase 2 (Homo sapiens (Human)) | BDBM148700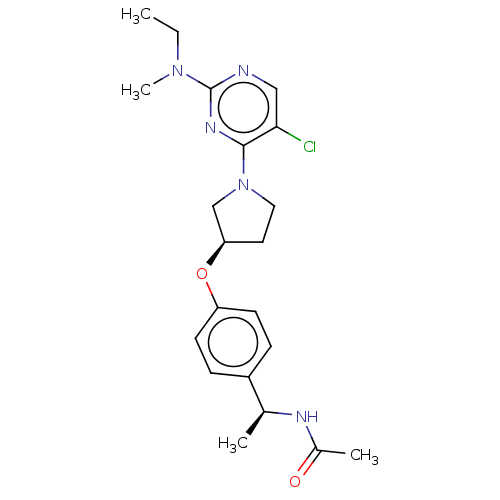 (US8962641, 8.56) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 60 | n/a | n/a | n/a | n/a | 7.5 | n/a |
Boehringer Ingelheim International GmbH US Patent | Assay Description Malonyl CoA formation by acetyl CoA carboxylases is stoichometrically linked to the consumption of ATP. ACC2 activity is measured in a NADH-linked ki... | US Patent US8962641 (2015) BindingDB Entry DOI: 10.7270/Q2H130Q5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetyl-CoA carboxylase 2 (Homo sapiens (Human)) | BDBM148954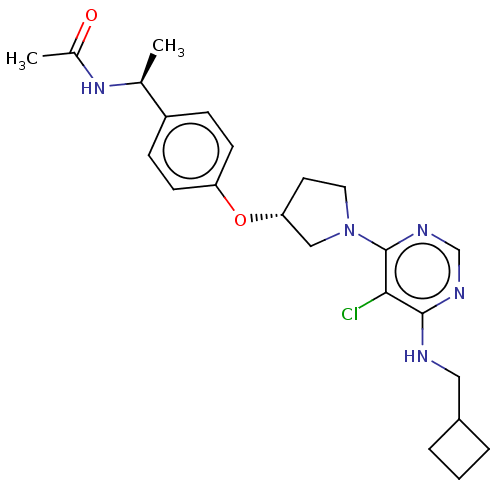 (US8962641, 8.311) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 62 | n/a | n/a | n/a | n/a | 7.5 | n/a |
Boehringer Ingelheim International GmbH US Patent | Assay Description Malonyl CoA formation by acetyl CoA carboxylases is stoichometrically linked to the consumption of ATP. ACC2 activity is measured in a NADH-linked ki... | US Patent US8962641 (2015) BindingDB Entry DOI: 10.7270/Q2H130Q5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetyl-CoA carboxylase 2 (Homo sapiens (Human)) | BDBM148497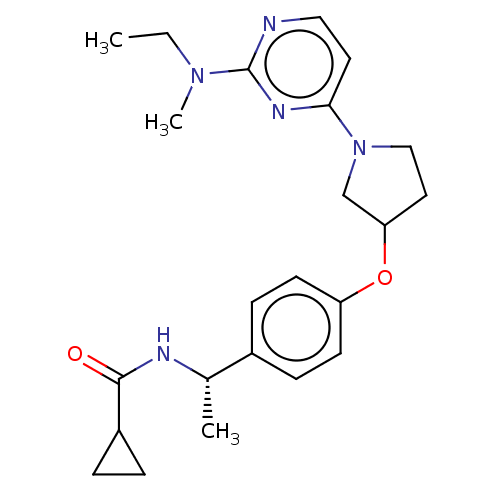 (US8962641, 2.14) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 63 | n/a | n/a | n/a | n/a | 7.5 | n/a |
Boehringer Ingelheim International GmbH US Patent | Assay Description Malonyl CoA formation by acetyl CoA carboxylases is stoichometrically linked to the consumption of ATP. ACC2 activity is measured in a NADH-linked ki... | US Patent US8962641 (2015) BindingDB Entry DOI: 10.7270/Q2H130Q5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 490 total ) | Next | Last >> |