

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| ALK tyrosine kinase receptor (Homo sapiens (Human)) | BDBM158154 (US10081622, Compound 11 | US10370379, Entrectinib ...) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid PDB UniChem Similars | PDB US Patent | n/a | n/a | 55 | n/a | n/a | n/a | n/a | 7.5 | n/a |
Nerviano Medical Sciences S.R.L. US Patent | Assay Description ALK enzyme needs pre-activation in order to linearize reaction kinetics.Kinase Buffer (KB) for ALKKinase buffer was composed of 50 mM HEPES pH 7.5 co... | US Patent US9029356 (2015) BindingDB Entry DOI: 10.7270/Q29C6W5G | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| ALK tyrosine kinase receptor (Homo sapiens (Human)) | BDBM158155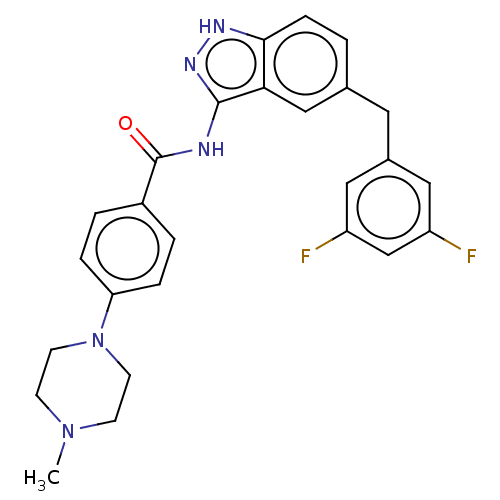 (US10081622, Compound 4 | US9029356, 4 | US9255087,...) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 207 | n/a | n/a | n/a | n/a | 7.5 | n/a |
Nerviano Medical Sciences S.R.L. US Patent | Assay Description ALK enzyme needs pre-activation in order to linearize reaction kinetics.Kinase Buffer (KB) for ALKKinase buffer was composed of 50 mM HEPES pH 7.5 co... | US Patent US9029356 (2015) BindingDB Entry DOI: 10.7270/Q29C6W5G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Insulin-like growth factor 1 receptor (Homo sapiens (Human)) | BDBM158154 (US10081622, Compound 11 | US10370379, Entrectinib ...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid PDB UniChem Similars | US Patent | n/a | n/a | 263 | n/a | n/a | n/a | n/a | n/a | n/a |
Nerviano Medical Sciences S.R.L. US Patent | Assay Description The inhibitory activity of putative kinase inhibitors and the potency of selected compounds were determined using a trans-phosphorylation assay.A spe... | US Patent US9029356 (2015) BindingDB Entry DOI: 10.7270/Q29C6W5G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aurora kinase B (Homo sapiens (Human)) | BDBM158154 (US10081622, Compound 11 | US10370379, Entrectinib ...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid PDB UniChem Similars | US Patent | n/a | n/a | 338 | n/a | n/a | n/a | n/a | n/a | n/a |
Nerviano Medical Sciences S.R.L. US Patent | Assay Description The inhibitory activity of putative kinase inhibitors and the potency of selected compounds were determined using a trans-phosphorylation assay.A spe... | US Patent US9029356 (2015) BindingDB Entry DOI: 10.7270/Q29C6W5G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| ALK tyrosine kinase receptor (Homo sapiens (Human)) | BDBM158156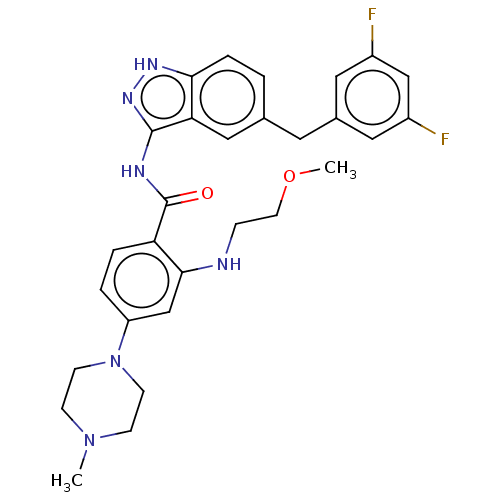 (US10081622, Compound 26 | US9029356, 26 | US925508...) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 411 | n/a | n/a | n/a | n/a | 7.5 | n/a |
Nerviano Medical Sciences S.R.L. US Patent | Assay Description ALK enzyme needs pre-activation in order to linearize reaction kinetics.Kinase Buffer (KB) for ALKKinase buffer was composed of 50 mM HEPES pH 7.5 co... | US Patent US9029356 (2015) BindingDB Entry DOI: 10.7270/Q29C6W5G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aurora kinase B (Homo sapiens (Human)) | BDBM158155 (US10081622, Compound 4 | US9029356, 4 | US9255087,...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 484 | n/a | n/a | n/a | n/a | n/a | n/a |
Nerviano Medical Sciences S.R.L. US Patent | Assay Description The inhibitory activity of putative kinase inhibitors and the potency of selected compounds were determined using a trans-phosphorylation assay.A spe... | US Patent US9029356 (2015) BindingDB Entry DOI: 10.7270/Q29C6W5G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aurora kinase B (Homo sapiens (Human)) | BDBM158156 (US10081622, Compound 26 | US9029356, 26 | US925508...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 568 | n/a | n/a | n/a | n/a | n/a | n/a |
Nerviano Medical Sciences S.R.L. US Patent | Assay Description The inhibitory activity of putative kinase inhibitors and the potency of selected compounds were determined using a trans-phosphorylation assay.A spe... | US Patent US9029356 (2015) BindingDB Entry DOI: 10.7270/Q29C6W5G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Insulin-like growth factor 1 receptor (Homo sapiens (Human)) | BDBM158156 (US10081622, Compound 26 | US9029356, 26 | US925508...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 1.10E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Nerviano Medical Sciences S.R.L. US Patent | Assay Description The inhibitory activity of putative kinase inhibitors and the potency of selected compounds were determined using a trans-phosphorylation assay.A spe... | US Patent US9029356 (2015) BindingDB Entry DOI: 10.7270/Q29C6W5G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| ALK tyrosine kinase receptor (Homo sapiens (Human)) | BDBM158157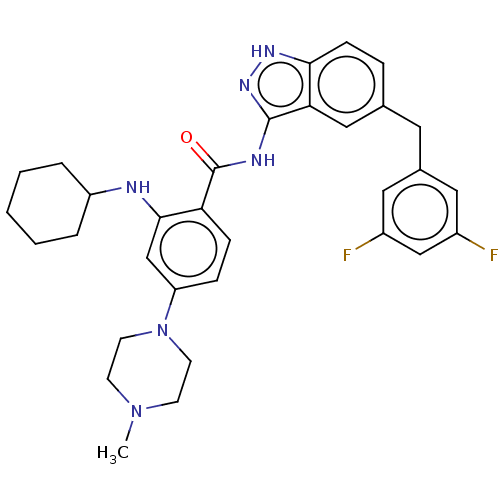 (US10081622, Compound 18 | US9029356, 18 | US925508...) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 1.77E+3 | n/a | n/a | n/a | n/a | 7.5 | n/a |
Nerviano Medical Sciences S.R.L. US Patent | Assay Description ALK enzyme needs pre-activation in order to linearize reaction kinetics.Kinase Buffer (KB) for ALKKinase buffer was composed of 50 mM HEPES pH 7.5 co... | US Patent US9029356 (2015) BindingDB Entry DOI: 10.7270/Q29C6W5G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Insulin-like growth factor 1 receptor (Homo sapiens (Human)) | BDBM158155 (US10081622, Compound 4 | US9029356, 4 | US9255087,...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 2.35E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Nerviano Medical Sciences S.R.L. US Patent | Assay Description The inhibitory activity of putative kinase inhibitors and the potency of selected compounds were determined using a trans-phosphorylation assay.A spe... | US Patent US9029356 (2015) BindingDB Entry DOI: 10.7270/Q29C6W5G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aurora kinase B (Homo sapiens (Human)) | BDBM158157 (US10081622, Compound 18 | US9029356, 18 | US925508...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 3.23E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Nerviano Medical Sciences S.R.L. US Patent | Assay Description The inhibitory activity of putative kinase inhibitors and the potency of selected compounds were determined using a trans-phosphorylation assay.A spe... | US Patent US9029356 (2015) BindingDB Entry DOI: 10.7270/Q29C6W5G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Insulin-like growth factor 1 receptor (Homo sapiens (Human)) | BDBM158157 (US10081622, Compound 18 | US9029356, 18 | US925508...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 6.07E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Nerviano Medical Sciences S.R.L. US Patent | Assay Description The inhibitory activity of putative kinase inhibitors and the potency of selected compounds were determined using a trans-phosphorylation assay.A spe... | US Patent US9029356 (2015) BindingDB Entry DOI: 10.7270/Q29C6W5G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||