

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
BDBM158157 US10081622, Compound 18::US9029356, 18::US9255087, 18::US9616059, 18
SMILES: CN1CCN(CC1)c1ccc(C(=O)Nc2n[nH]c3ccc(Cc4cc(F)cc(F)c4)cc23)c(NC2CCCCC2)c1
InChI Key: InChIKey=VHSQRCDULUNORA-UHFFFAOYSA-N
Data: 14 IC50
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kcal/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| ALK tyrosine kinase receptor (Homo sapiens (Human)) | BDBM158157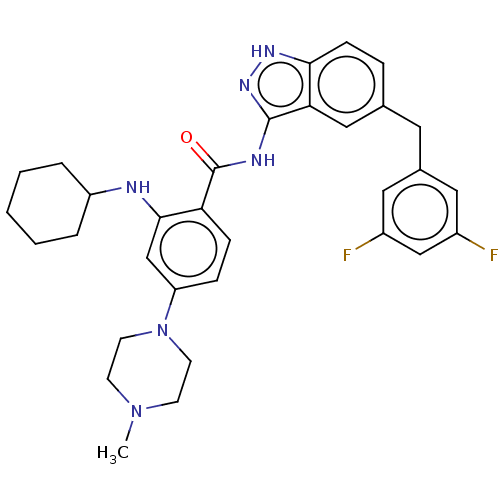 (US10081622, Compound 18 | US9029356, 18 | US925508...) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 1.77E+3 | n/a | n/a | n/a | n/a | 7.5 | n/a |
Nerviano Medical Sciences S.R.L. US Patent | Assay Description ALK enzyme needs pre-activation in order to linearize reaction kinetics.Kinase Buffer (KB) for ALKKinase buffer was composed of 50 mM HEPES pH 7.5 co... | US Patent US9029356 (2015) BindingDB Entry DOI: 10.7270/Q29C6W5G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Insulin-like growth factor I receptor (Homo sapiens (Human)) | BDBM158157 (US10081622, Compound 18 | US9029356, 18 | US925508...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 6.07E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Nerviano Medical Sciences S.R.L. US Patent | Assay Description The inhibitory activity of putative kinase inhibitors and the potency of selected compounds were determined using a trans-phosphorylation assay.A spe... | US Patent US9029356 (2015) BindingDB Entry DOI: 10.7270/Q29C6W5G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aurora kinase B (Homo sapiens (Human)) | BDBM158157 (US10081622, Compound 18 | US9029356, 18 | US925508...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 3.23E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Nerviano Medical Sciences S.R.L. US Patent | Assay Description The inhibitory activity of putative kinase inhibitors and the potency of selected compounds were determined using a trans-phosphorylation assay.A spe... | US Patent US9029356 (2015) BindingDB Entry DOI: 10.7270/Q29C6W5G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| ALK tyrosine kinase receptor (Homo sapiens (Human)) | BDBM158157 (US10081622, Compound 18 | US9029356, 18 | US925508...) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 1.77E+3 | n/a | n/a | n/a | n/a | 7.5 | n/a |
NERVIANO MEDICAL SCIENCES S.R.L. US Patent | Assay Description BioKinase buffer was composed of 50 mM HEPES pH 7.5 containing 1 mM MnCl2, 5 mM MgCl2, 1 mM DTT, 3 microM Na3VO4, and 0.2 mg/mL BSA. 3x KB is buffer ... | US Patent US9255087 (2016) BindingDB Entry DOI: 10.7270/Q20G3J06 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Insulin-like growth factor I receptor (Homo sapiens (Human)) | BDBM158157 (US10081622, Compound 18 | US9029356, 18 | US925508...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 6.07E+3 | n/a | n/a | n/a | n/a | 7.9 | 28 |
NERVIANO MEDICAL SCIENCES S.R.L. US Patent | Assay Description Kinase buffer was composed of 50 mM HEPES pH 7.9 containing 3 mM MnCl2, 1 mM DTT, 3 microM Na3VO4, and 0.2 mg/mL BSA. 3x KB is buffer of the same com... | US Patent US9255087 (2016) BindingDB Entry DOI: 10.7270/Q20G3J06 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aurora kinase A (Homo sapiens (Human)) | BDBM158157 (US10081622, Compound 18 | US9029356, 18 | US925508...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 3.23E+3 | n/a | n/a | n/a | n/a | 7.0 | n/a |
NERVIANO MEDICAL SCIENCES S.R.L. US Patent | Assay Description The kinase buffer was composed of 50 mM HEPES, pH 7.0, 10 mM MnCl2, 1 mM DTT, 3 microM Na3VO4, and 0.2 mg/mL BSA. The kinase assay was run with an en... | US Patent US9255087 (2016) BindingDB Entry DOI: 10.7270/Q20G3J06 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aurora kinase A (Homo sapiens (Human)) | BDBM158157 (US10081622, Compound 18 | US9029356, 18 | US925508...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 3.23E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
NERVIANO MEDICAL SCIENCES S.R.L. US Patent | Assay Description i. Kinase Buffer (KB) for Aurora-2The kinase buffer was composed of 50 mM HEPES, pH 7.0, 10 mM MnCl2, 1 mM DTT, 3 microM Na3VO4, and 0.2 mg/mL BSA.ii... | US Patent US9616059 (2017) BindingDB Entry DOI: 10.7270/Q2TT4T0S | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| ALK tyrosine kinase receptor (Homo sapiens (Human)) | BDBM158157 (US10081622, Compound 18 | US9029356, 18 | US925508...) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 560 | n/a | n/a | n/a | n/a | n/a | n/a |
Nerviano Medical Sciences Srl Curated by ChEMBL | Assay Description Inhibition of recombinant ALK (unknown origin) in presence of gamma33-ATP | J Med Chem 59: 3392-408 (2016) BindingDB Entry DOI: 10.7270/Q27M09TG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| ALK tyrosine kinase receptor (Homo sapiens (Human)) | BDBM158157 (US10081622, Compound 18 | US9029356, 18 | US925508...) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 1.77E+3 | n/a | n/a | n/a | n/a | 7.5 | n/a |
NERVIANO MEDICAL SCIENCES S.R.L. US Patent | Assay Description ALK enzyme needs pre-activation in order to linearize reaction kinetics.i. Kinase Buffer (KB) for ALK.Kinase buffer was composed of 50 mM HEPES pH 7.... | US Patent US10081622 (2018) BindingDB Entry DOI: 10.7270/Q2833V2H | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Insulin-like growth factor I receptor (Homo sapiens (Human)) | BDBM158157 (US10081622, Compound 18 | US9029356, 18 | US925508...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 6.07E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
NERVIANO MEDICAL SCIENCES S.R.L. US Patent | Assay Description The inhibitory activity of putative kinase inhibitors and the potency of selected compounds were determined using a trans-phosphorylation assay.A spe... | US Patent US10081622 (2018) BindingDB Entry DOI: 10.7270/Q2833V2H | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aurora kinase A (Homo sapiens (Human)) | BDBM158157 (US10081622, Compound 18 | US9029356, 18 | US925508...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 3.23E+3 | n/a | n/a | n/a | n/a | 7.0 | n/a |
NERVIANO MEDICAL SCIENCES S.R.L. US Patent | Assay Description The in vitro kinase inhibition assay was conducted in the same way as described for IGF-1R. At variance with IGF-1R, Aurora-2 enzyme does not need pr... | US Patent US10081622 (2018) BindingDB Entry DOI: 10.7270/Q2833V2H | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| ALK tyrosine kinase receptor (Homo sapiens (Human)) | BDBM158157 (US10081622, Compound 18 | US9029356, 18 | US925508...) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 1.77E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
NERVIANO MEDICAL SCIENCES S.R.L. US Patent | Assay Description Kinase Buffer (KB) for ALKKinase buffer was composed of 50 mM HEPES pH 7.5 containing 1 mM MnCl2, mM MgCl2, 1 mM DTT, 3 microM Na3VO4, and 0.2 mg/mL ... | US Patent US9616059 (2017) BindingDB Entry DOI: 10.7270/Q2TT4T0S | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Insulin-like growth factor I receptor (Homo sapiens (Human)) | BDBM158157 (US10081622, Compound 18 | US9029356, 18 | US925508...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 6.07E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
NERVIANO MEDICAL SCIENCES S.R.L. US Patent | Assay Description Kinase buffer was composed of 50 mM HEPES pH 7.9 containing 3 mM MnCl2, 1 mM DTT, 3 microM Na3VO4, and 0.2 mg/mL BSA. 3×KB is buffer of the same comp... | US Patent US9616059 (2017) BindingDB Entry DOI: 10.7270/Q2TT4T0S | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Insulin receptor (Homo sapiens (Human)) | BDBM158157 (US10081622, Compound 18 | US9029356, 18 | US925508...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 3.82E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Nerviano Medical Sciences Srl Curated by ChEMBL | Assay Description Inhibition of IR (unknown origin) in presence of gamma33-ATP | J Med Chem 59: 3392-408 (2016) BindingDB Entry DOI: 10.7270/Q27M09TG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||