

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Histone deacetylase 6 (Homo sapiens (Human)) | BDBM191641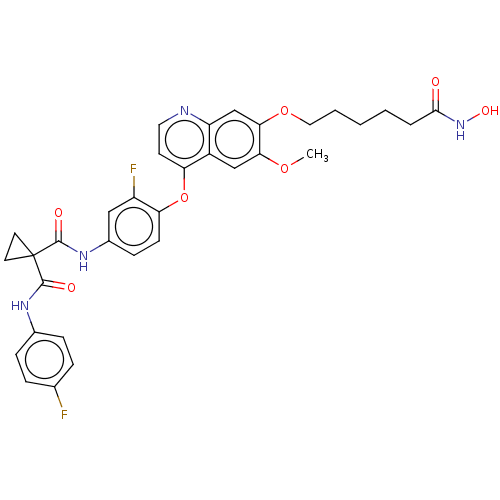 (US9186318, 13) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 8.30 | n/a | n/a | n/a | n/a | n/a | n/a |
Beijing Konruns Pharmaceutical Co., Ltd US Patent | Assay Description The inhibitions of activities of HDAC enzymes by the compounds N1'-[3-fluoro-4-[[7-[4-(hydroxyamino)-4-oxobutoxy]-6-methoxy-4-quinolyl]oxy]phenyl]-N1... | US Patent US9186318 (2015) BindingDB Entry DOI: 10.7270/Q2ZP44XG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Vascular endothelial growth factor receptor 2 (Homo sapiens (Human)) | BDBM191640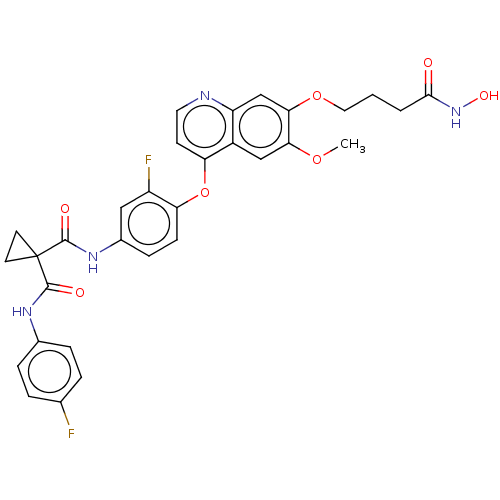 (US9186318, 9) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 12 | n/a | n/a | n/a | n/a | n/a | n/a |
Beijing Konruns Pharmaceutical Co., Ltd US Patent | Assay Description This example shows that the inhibitory activity of the compound, N1'-[3-fluoro-4-[[7-[4-(hydroxyamino)-4-oxobutoxy]-6-methoxy-4-quinolyl]oxy]phenyl]-... | US Patent US9186318 (2015) BindingDB Entry DOI: 10.7270/Q2ZP44XG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase receptor UFO (Homo sapiens (Human)) | BDBM191640 (US9186318, 9) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 19 | n/a | n/a | n/a | n/a | n/a | n/a |
Beijing Konruns Pharmaceutical Co., Ltd US Patent | Assay Description This example shows that the inhibitory activity of the compound, N1'-[3-fluoro-4-[[7-[4-(hydroxyamino)-4-oxobutoxy]-6-methoxy-4-quinolyl]oxy]phenyl]-... | US Patent US9186318 (2015) BindingDB Entry DOI: 10.7270/Q2ZP44XG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Receptor-type tyrosine-protein kinase FLT3 (Homo sapiens (Human)) | BDBM191640 (US9186318, 9) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 31 | n/a | n/a | n/a | n/a | n/a | n/a |
Beijing Konruns Pharmaceutical Co., Ltd US Patent | Assay Description This example shows that the inhibitory activity of the compound, N1'-[3-fluoro-4-[[7-[4-(hydroxyamino)-4-oxobutoxy]-6-methoxy-4-quinolyl]oxy]phenyl]-... | US Patent US9186318 (2015) BindingDB Entry DOI: 10.7270/Q2ZP44XG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mast/stem cell growth factor receptor Kit (Homo sapiens (Human)) | BDBM191640 (US9186318, 9) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 115 | n/a | n/a | n/a | n/a | n/a | n/a |
Beijing Konruns Pharmaceutical Co., Ltd US Patent | Assay Description This example shows that the inhibitory activity of the compound, N1'-[3-fluoro-4-[[7-[4-(hydroxyamino)-4-oxobutoxy]-6-methoxy-4-quinolyl]oxy]phenyl]-... | US Patent US9186318 (2015) BindingDB Entry DOI: 10.7270/Q2ZP44XG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Hepatocyte growth factor receptor (Homo sapiens (Human)) | BDBM191640 (US9186318, 9) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 119 | n/a | n/a | n/a | n/a | n/a | n/a |
Beijing Konruns Pharmaceutical Co., Ltd US Patent | Assay Description This example shows that the inhibitory activity of the compound, N1'-[3-fluoro-4-[[7-[4-(hydroxyamino)-4-oxobutoxy]-6-methoxy-4-quinolyl]oxy]phenyl]-... | US Patent US9186318 (2015) BindingDB Entry DOI: 10.7270/Q2ZP44XG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Proto-oncogene tyrosine-protein kinase receptor Ret (Homo sapiens (Human)) | BDBM191640 (US9186318, 9) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 154 | n/a | n/a | n/a | n/a | n/a | n/a |
Beijing Konruns Pharmaceutical Co., Ltd US Patent | Assay Description This example shows that the inhibitory activity of the compound, N1'-[3-fluoro-4-[[7-[4-(hydroxyamino)-4-oxobutoxy]-6-methoxy-4-quinolyl]oxy]phenyl]-... | US Patent US9186318 (2015) BindingDB Entry DOI: 10.7270/Q2ZP44XG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Platelet-derived growth factor receptor beta (Homo sapiens (Human)) | BDBM191640 (US9186318, 9) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 172 | n/a | n/a | n/a | n/a | n/a | n/a |
Beijing Konruns Pharmaceutical Co., Ltd US Patent | Assay Description This example shows that the inhibitory activity of the compound, N1'-[3-fluoro-4-[[7-[4-(hydroxyamino)-4-oxobutoxy]-6-methoxy-4-quinolyl]oxy]phenyl]-... | US Patent US9186318 (2015) BindingDB Entry DOI: 10.7270/Q2ZP44XG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone deacetylase 6 (Homo sapiens (Human)) | BDBM191640 (US9186318, 9) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 242 | n/a | n/a | n/a | n/a | n/a | n/a |
Beijing Konruns Pharmaceutical Co., Ltd US Patent | Assay Description The inhibitions of activities of HDAC enzymes by the compounds N1'-[3-fluoro-4-[[7-[4-(hydroxyamino)-4-oxobutoxy]-6-methoxy-4-quinolyl]oxy]phenyl]-N1... | US Patent US9186318 (2015) BindingDB Entry DOI: 10.7270/Q2ZP44XG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone deacetylase 2 (Homo sapiens (Human)) | BDBM191640 (US9186318, 9) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 431 | n/a | n/a | n/a | n/a | n/a | n/a |
Beijing Konruns Pharmaceutical Co., Ltd US Patent | Assay Description The inhibitions of activities of HDAC enzymes by the compounds N1'-[3-fluoro-4-[[7-[4-(hydroxyamino)-4-oxobutoxy]-6-methoxy-4-quinolyl]oxy]phenyl]-N1... | US Patent US9186318 (2015) BindingDB Entry DOI: 10.7270/Q2ZP44XG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Proto-oncogene tyrosine-protein kinase Src (Homo sapiens (Human)) | BDBM191640 (US9186318, 9) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | >630 | n/a | n/a | n/a | n/a | n/a | n/a |
Beijing Konruns Pharmaceutical Co., Ltd US Patent | Assay Description This example shows that the inhibitory activity of the compound, N1'-[3-fluoro-4-[[7-[4-(hydroxyamino)-4-oxobutoxy]-6-methoxy-4-quinolyl]oxy]phenyl]-... | US Patent US9186318 (2015) BindingDB Entry DOI: 10.7270/Q2ZP44XG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| ALK tyrosine kinase receptor (Homo sapiens (Human)) | BDBM191640 (US9186318, 9) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | >630 | n/a | n/a | n/a | n/a | n/a | n/a |
Beijing Konruns Pharmaceutical Co., Ltd US Patent | Assay Description This example shows that the inhibitory activity of the compound, N1'-[3-fluoro-4-[[7-[4-(hydroxyamino)-4-oxobutoxy]-6-methoxy-4-quinolyl]oxy]phenyl]-... | US Patent US9186318 (2015) BindingDB Entry DOI: 10.7270/Q2ZP44XG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone deacetylase 8 (Homo sapiens (Human)) | BDBM191641 (US9186318, 13) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 1.63E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Beijing Konruns Pharmaceutical Co., Ltd US Patent | Assay Description The inhibitions of activities of HDAC enzymes by the compounds N1'-[3-fluoro-4-[[7-[4-(hydroxyamino)-4-oxobutoxy]-6-methoxy-4-quinolyl]oxy]phenyl]-N1... | US Patent US9186318 (2015) BindingDB Entry DOI: 10.7270/Q2ZP44XG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone deacetylase 2 (Homo sapiens (Human)) | BDBM191641 (US9186318, 13) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 2.74E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Beijing Konruns Pharmaceutical Co., Ltd US Patent | Assay Description The inhibitions of activities of HDAC enzymes by the compounds N1'-[3-fluoro-4-[[7-[4-(hydroxyamino)-4-oxobutoxy]-6-methoxy-4-quinolyl]oxy]phenyl]-N1... | US Patent US9186318 (2015) BindingDB Entry DOI: 10.7270/Q2ZP44XG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone deacetylase 11 (Homo sapiens (Human)) | BDBM191641 (US9186318, 13) | NCI pathway Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 6.13E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Beijing Konruns Pharmaceutical Co., Ltd US Patent | Assay Description The inhibitions of activities of HDAC enzymes by the compounds N1'-[3-fluoro-4-[[7-[4-(hydroxyamino)-4-oxobutoxy]-6-methoxy-4-quinolyl]oxy]phenyl]-N1... | US Patent US9186318 (2015) BindingDB Entry DOI: 10.7270/Q2ZP44XG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone deacetylase 8 (Homo sapiens (Human)) | BDBM191640 (US9186318, 9) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 8.94E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Beijing Konruns Pharmaceutical Co., Ltd US Patent | Assay Description The inhibitions of activities of HDAC enzymes by the compounds N1'-[3-fluoro-4-[[7-[4-(hydroxyamino)-4-oxobutoxy]-6-methoxy-4-quinolyl]oxy]phenyl]-N1... | US Patent US9186318 (2015) BindingDB Entry DOI: 10.7270/Q2ZP44XG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone deacetylase 4 (Homo sapiens (Human)) | BDBM191640 (US9186318, 9) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Beijing Konruns Pharmaceutical Co., Ltd US Patent | Assay Description The inhibitions of activities of HDAC enzymes by the compounds N1'-[3-fluoro-4-[[7-[4-(hydroxyamino)-4-oxobutoxy]-6-methoxy-4-quinolyl]oxy]phenyl]-N1... | US Patent US9186318 (2015) BindingDB Entry DOI: 10.7270/Q2ZP44XG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Polyamine deacetylase HDAC10 (Homo sapiens (Human)) | BDBM191640 (US9186318, 9) | NCI pathway Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Beijing Konruns Pharmaceutical Co., Ltd US Patent | Assay Description The inhibitions of activities of HDAC enzymes by the compounds N1'-[3-fluoro-4-[[7-[4-(hydroxyamino)-4-oxobutoxy]-6-methoxy-4-quinolyl]oxy]phenyl]-N1... | US Patent US9186318 (2015) BindingDB Entry DOI: 10.7270/Q2ZP44XG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone deacetylase 5 (Homo sapiens (Human)) | BDBM191641 (US9186318, 13) | KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Beijing Konruns Pharmaceutical Co., Ltd US Patent | Assay Description The inhibitions of activities of HDAC enzymes by the compounds N1'-[3-fluoro-4-[[7-[4-(hydroxyamino)-4-oxobutoxy]-6-methoxy-4-quinolyl]oxy]phenyl]-N1... | US Patent US9186318 (2015) BindingDB Entry DOI: 10.7270/Q2ZP44XG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone deacetylase 7 (Homo sapiens (Human)) | BDBM191640 (US9186318, 9) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Beijing Konruns Pharmaceutical Co., Ltd US Patent | Assay Description The inhibitions of activities of HDAC enzymes by the compounds N1'-[3-fluoro-4-[[7-[4-(hydroxyamino)-4-oxobutoxy]-6-methoxy-4-quinolyl]oxy]phenyl]-N1... | US Patent US9186318 (2015) BindingDB Entry DOI: 10.7270/Q2ZP44XG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone deacetylase 9 (Homo sapiens (Human)) | BDBM191641 (US9186318, 13) | Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Beijing Konruns Pharmaceutical Co., Ltd US Patent | Assay Description The inhibitions of activities of HDAC enzymes by the compounds N1'-[3-fluoro-4-[[7-[4-(hydroxyamino)-4-oxobutoxy]-6-methoxy-4-quinolyl]oxy]phenyl]-N1... | US Patent US9186318 (2015) BindingDB Entry DOI: 10.7270/Q2ZP44XG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone deacetylase 3 (Homo sapiens (Human)) | BDBM191640 (US9186318, 9) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Beijing Konruns Pharmaceutical Co., Ltd US Patent | Assay Description The inhibitions of activities of HDAC enzymes by the compounds N1'-[3-fluoro-4-[[7-[4-(hydroxyamino)-4-oxobutoxy]-6-methoxy-4-quinolyl]oxy]phenyl]-N1... | US Patent US9186318 (2015) BindingDB Entry DOI: 10.7270/Q2ZP44XG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone deacetylase 1 (Homo sapiens (Human)) | BDBM191641 (US9186318, 13) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Beijing Konruns Pharmaceutical Co., Ltd US Patent | Assay Description The inhibitions of activities of HDAC enzymes by the compounds N1'-[3-fluoro-4-[[7-[4-(hydroxyamino)-4-oxobutoxy]-6-methoxy-4-quinolyl]oxy]phenyl]-N1... | US Patent US9186318 (2015) BindingDB Entry DOI: 10.7270/Q2ZP44XG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone deacetylase 11 (Homo sapiens (Human)) | BDBM191640 (US9186318, 9) | NCI pathway Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Beijing Konruns Pharmaceutical Co., Ltd US Patent | Assay Description The inhibitions of activities of HDAC enzymes by the compounds N1'-[3-fluoro-4-[[7-[4-(hydroxyamino)-4-oxobutoxy]-6-methoxy-4-quinolyl]oxy]phenyl]-N1... | US Patent US9186318 (2015) BindingDB Entry DOI: 10.7270/Q2ZP44XG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone deacetylase 9 (Homo sapiens (Human)) | BDBM191640 (US9186318, 9) | Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Beijing Konruns Pharmaceutical Co., Ltd US Patent | Assay Description The inhibitions of activities of HDAC enzymes by the compounds N1'-[3-fluoro-4-[[7-[4-(hydroxyamino)-4-oxobutoxy]-6-methoxy-4-quinolyl]oxy]phenyl]-N1... | US Patent US9186318 (2015) BindingDB Entry DOI: 10.7270/Q2ZP44XG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone deacetylase 5 (Homo sapiens (Human)) | BDBM191640 (US9186318, 9) | KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Beijing Konruns Pharmaceutical Co., Ltd US Patent | Assay Description The inhibitions of activities of HDAC enzymes by the compounds N1'-[3-fluoro-4-[[7-[4-(hydroxyamino)-4-oxobutoxy]-6-methoxy-4-quinolyl]oxy]phenyl]-N1... | US Patent US9186318 (2015) BindingDB Entry DOI: 10.7270/Q2ZP44XG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone deacetylase 1 (Homo sapiens (Human)) | BDBM191640 (US9186318, 9) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Beijing Konruns Pharmaceutical Co., Ltd US Patent | Assay Description The inhibitions of activities of HDAC enzymes by the compounds N1'-[3-fluoro-4-[[7-[4-(hydroxyamino)-4-oxobutoxy]-6-methoxy-4-quinolyl]oxy]phenyl]-N1... | US Patent US9186318 (2015) BindingDB Entry DOI: 10.7270/Q2ZP44XG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone deacetylase 7 (Homo sapiens (Human)) | BDBM191641 (US9186318, 13) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Beijing Konruns Pharmaceutical Co., Ltd US Patent | Assay Description The inhibitions of activities of HDAC enzymes by the compounds N1'-[3-fluoro-4-[[7-[4-(hydroxyamino)-4-oxobutoxy]-6-methoxy-4-quinolyl]oxy]phenyl]-N1... | US Patent US9186318 (2015) BindingDB Entry DOI: 10.7270/Q2ZP44XG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone deacetylase 3 (Homo sapiens (Human)) | BDBM191641 (US9186318, 13) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Beijing Konruns Pharmaceutical Co., Ltd US Patent | Assay Description The inhibitions of activities of HDAC enzymes by the compounds N1'-[3-fluoro-4-[[7-[4-(hydroxyamino)-4-oxobutoxy]-6-methoxy-4-quinolyl]oxy]phenyl]-N1... | US Patent US9186318 (2015) BindingDB Entry DOI: 10.7270/Q2ZP44XG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone deacetylase 4 (Homo sapiens (Human)) | BDBM191641 (US9186318, 13) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Beijing Konruns Pharmaceutical Co., Ltd US Patent | Assay Description The inhibitions of activities of HDAC enzymes by the compounds N1'-[3-fluoro-4-[[7-[4-(hydroxyamino)-4-oxobutoxy]-6-methoxy-4-quinolyl]oxy]phenyl]-N1... | US Patent US9186318 (2015) BindingDB Entry DOI: 10.7270/Q2ZP44XG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Polyamine deacetylase HDAC10 (Homo sapiens (Human)) | BDBM191641 (US9186318, 13) | NCI pathway Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 1.19E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Beijing Konruns Pharmaceutical Co., Ltd US Patent | Assay Description The inhibitions of activities of HDAC enzymes by the compounds N1'-[3-fluoro-4-[[7-[4-(hydroxyamino)-4-oxobutoxy]-6-methoxy-4-quinolyl]oxy]phenyl]-N1... | US Patent US9186318 (2015) BindingDB Entry DOI: 10.7270/Q2ZP44XG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||