

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Prostaglandin G/H synthase 2 (Homo sapiens (Human)) | BDBM11639 (4-[5-(4-methylphenyl)-3-(trifluoromethyl)-1H-pyraz...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | DrugBank PDB US Patent | n/a | n/a | 500 | n/a | n/a | n/a | n/a | n/a | n/a |
German University; Southern Research Institute US Patent | Assay Description The activities of COX-1 and COX-2 were measured after the addition of arachidonic acid and incubation at 25° C. for 5 min by absorbance at 590 nm as ... | US Patent US9388139 (2016) BindingDB Entry DOI: 10.7270/Q2GF0SFS | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| cGMP-specific 3',5'-cyclic phosphodiesterase (Homo sapiens (Human)) | BDBM54632 (4-[3-(4-methoxyphenyl)-5-phenyl-3,4-dihydropyrazol...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 1.20E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
German University; Southern Research Institute US Patent | Assay Description PDE activity was measured using the IMAP fluorescence polarization assay (Molecular Devices) in which binding of hydrolyzed cyclic nucleotide substra... | US Patent US9388139 (2016) BindingDB Entry DOI: 10.7270/Q2GF0SFS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| cGMP-specific 3',5'-cyclic phosphodiesterase (Homo sapiens (Human)) | BDBM236641 (US9388139, 9) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 1.80E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
German University; Southern Research Institute US Patent | Assay Description PDE activity was measured using the IMAP fluorescence polarization assay (Molecular Devices) in which binding of hydrolyzed cyclic nucleotide substra... | US Patent US9388139 (2016) BindingDB Entry DOI: 10.7270/Q2GF0SFS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| cGMP-specific 3',5'-cyclic phosphodiesterase (Homo sapiens (Human)) | BDBM236648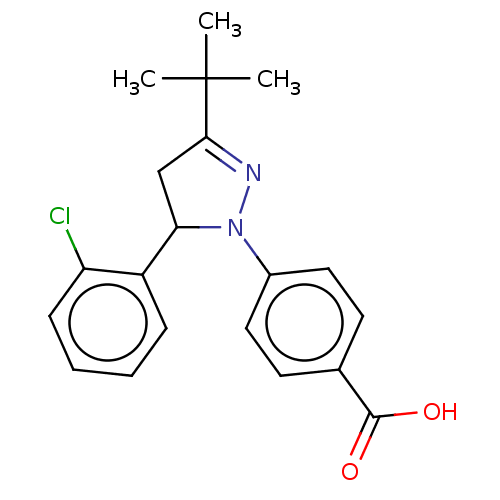 (US9388139, 17) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 1.90E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
German University; Southern Research Institute US Patent | Assay Description PDE activity was measured using the IMAP fluorescence polarization assay (Molecular Devices) in which binding of hydrolyzed cyclic nucleotide substra... | US Patent US9388139 (2016) BindingDB Entry DOI: 10.7270/Q2GF0SFS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| cGMP-specific 3',5'-cyclic phosphodiesterase (Homo sapiens (Human)) | BDBM236651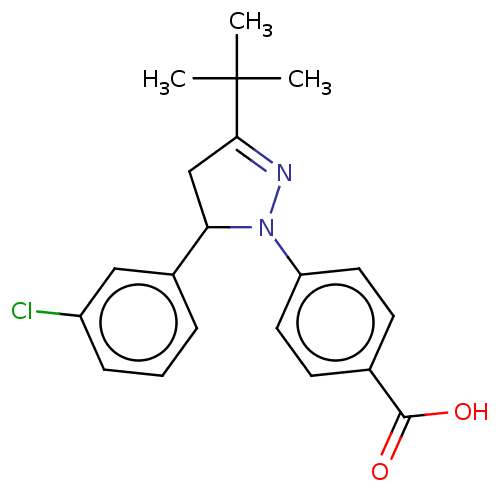 (US9388139, 20) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 2.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
German University; Southern Research Institute US Patent | Assay Description PDE activity was measured using the IMAP fluorescence polarization assay (Molecular Devices) in which binding of hydrolyzed cyclic nucleotide substra... | US Patent US9388139 (2016) BindingDB Entry DOI: 10.7270/Q2GF0SFS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| cGMP-specific 3',5'-cyclic phosphodiesterase (Homo sapiens (Human)) | BDBM236634 (US9388139, 1) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 2.02E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
German University; Southern Research Institute US Patent | Assay Description PDE activity was measured using the IMAP fluorescence polarization assay (Molecular Devices) in which binding of hydrolyzed cyclic nucleotide substra... | US Patent US9388139 (2016) BindingDB Entry DOI: 10.7270/Q2GF0SFS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| cGMP-specific 3',5'-cyclic phosphodiesterase (Homo sapiens (Human)) | BDBM236638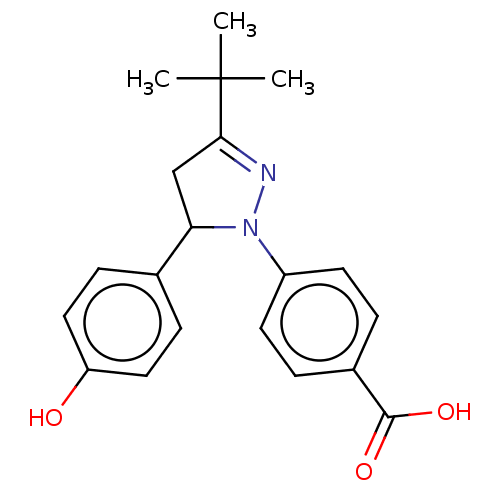 (US9388139, 5) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 2.10E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
German University; Southern Research Institute US Patent | Assay Description PDE activity was measured using the IMAP fluorescence polarization assay (Molecular Devices) in which binding of hydrolyzed cyclic nucleotide substra... | US Patent US9388139 (2016) BindingDB Entry DOI: 10.7270/Q2GF0SFS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| cGMP-specific 3',5'-cyclic phosphodiesterase (Homo sapiens (Human)) | BDBM236650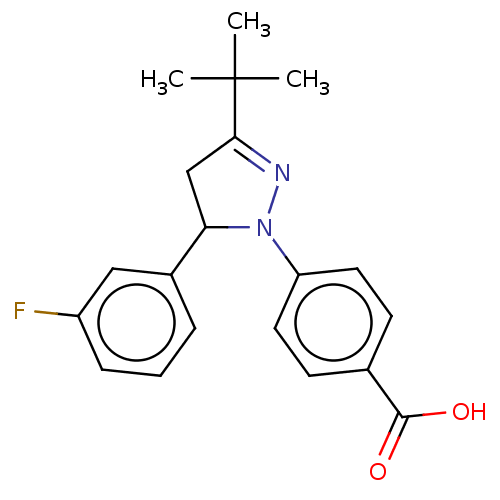 (US9388139, 19) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 2.70E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
German University; Southern Research Institute US Patent | Assay Description PDE activity was measured using the IMAP fluorescence polarization assay (Molecular Devices) in which binding of hydrolyzed cyclic nucleotide substra... | US Patent US9388139 (2016) BindingDB Entry DOI: 10.7270/Q2GF0SFS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| cGMP-specific 3',5'-cyclic phosphodiesterase (Homo sapiens (Human)) | BDBM236646 (US9388139, 15) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 2.90E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
German University; Southern Research Institute US Patent | Assay Description PDE activity was measured using the IMAP fluorescence polarization assay (Molecular Devices) in which binding of hydrolyzed cyclic nucleotide substra... | US Patent US9388139 (2016) BindingDB Entry DOI: 10.7270/Q2GF0SFS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| cGMP-specific 3',5'-cyclic phosphodiesterase (Homo sapiens (Human)) | BDBM236658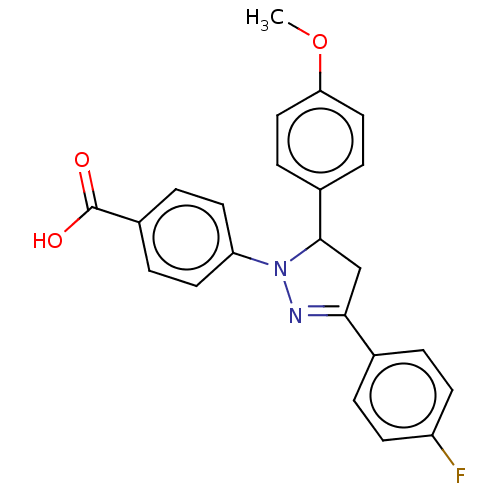 (US9388139, 27) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 3.10E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
German University; Southern Research Institute US Patent | Assay Description PDE activity was measured using the IMAP fluorescence polarization assay (Molecular Devices) in which binding of hydrolyzed cyclic nucleotide substra... | US Patent US9388139 (2016) BindingDB Entry DOI: 10.7270/Q2GF0SFS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| cGMP-specific 3',5'-cyclic phosphodiesterase (Homo sapiens (Human)) | BDBM236647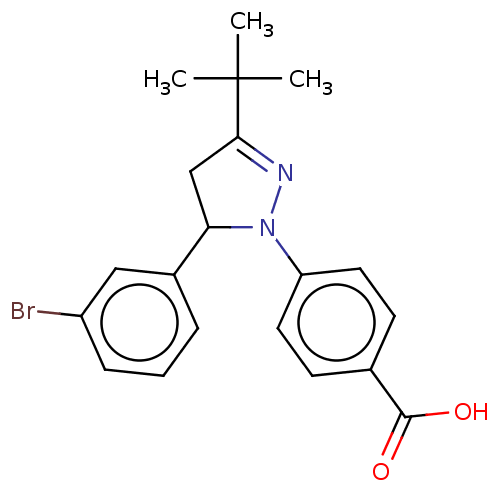 (US9388139, 16) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 3.10E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
German University; Southern Research Institute US Patent | Assay Description PDE activity was measured using the IMAP fluorescence polarization assay (Molecular Devices) in which binding of hydrolyzed cyclic nucleotide substra... | US Patent US9388139 (2016) BindingDB Entry DOI: 10.7270/Q2GF0SFS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| cGMP-specific 3',5'-cyclic phosphodiesterase (Homo sapiens (Human)) | BDBM236640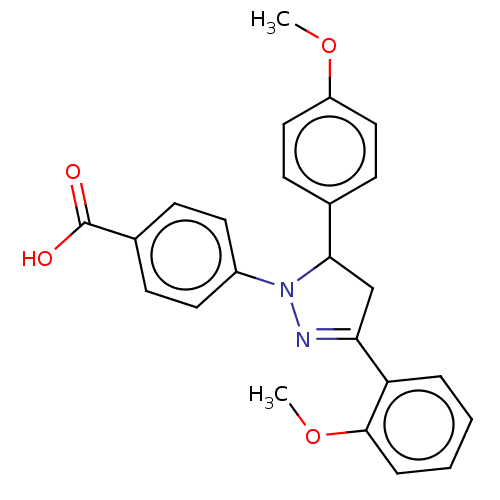 (US9388139, 8) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 3.20E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
German University; Southern Research Institute US Patent | Assay Description PDE activity was measured using the IMAP fluorescence polarization assay (Molecular Devices) in which binding of hydrolyzed cyclic nucleotide substra... | US Patent US9388139 (2016) BindingDB Entry DOI: 10.7270/Q2GF0SFS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| cGMP-specific 3',5'-cyclic phosphodiesterase (Homo sapiens (Human)) | BDBM236636 (US9388139, 3) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 3.20E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
German University; Southern Research Institute US Patent | Assay Description PDE activity was measured using the IMAP fluorescence polarization assay (Molecular Devices) in which binding of hydrolyzed cyclic nucleotide substra... | US Patent US9388139 (2016) BindingDB Entry DOI: 10.7270/Q2GF0SFS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| cGMP-specific 3',5'-cyclic phosphodiesterase (Homo sapiens (Human)) | BDBM236649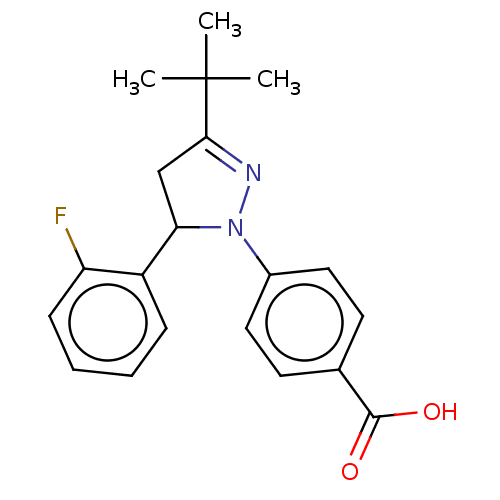 (US9388139, 18) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 3.30E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
German University; Southern Research Institute US Patent | Assay Description PDE activity was measured using the IMAP fluorescence polarization assay (Molecular Devices) in which binding of hydrolyzed cyclic nucleotide substra... | US Patent US9388139 (2016) BindingDB Entry DOI: 10.7270/Q2GF0SFS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| cGMP-specific 3',5'-cyclic phosphodiesterase (Homo sapiens (Human)) | BDBM236656 (US9388139, 25) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 3.30E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
German University; Southern Research Institute US Patent | Assay Description PDE activity was measured using the IMAP fluorescence polarization assay (Molecular Devices) in which binding of hydrolyzed cyclic nucleotide substra... | US Patent US9388139 (2016) BindingDB Entry DOI: 10.7270/Q2GF0SFS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| cGMP-specific 3',5'-cyclic phosphodiesterase (Homo sapiens (Human)) | BDBM236643 (US9388139, 12) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 3.70E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
German University; Southern Research Institute US Patent | Assay Description PDE activity was measured using the IMAP fluorescence polarization assay (Molecular Devices) in which binding of hydrolyzed cyclic nucleotide substra... | US Patent US9388139 (2016) BindingDB Entry DOI: 10.7270/Q2GF0SFS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| cGMP-specific 3',5'-cyclic phosphodiesterase (Homo sapiens (Human)) | BDBM43220 (4-[3-(4-methoxyphenyl)-5-phenyl-3,4-dihydropyrazol...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 3.80E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
German University; Southern Research Institute US Patent | Assay Description PDE activity was measured using the IMAP fluorescence polarization assay (Molecular Devices) in which binding of hydrolyzed cyclic nucleotide substra... | US Patent US9388139 (2016) BindingDB Entry DOI: 10.7270/Q2GF0SFS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| cGMP-specific 3',5'-cyclic phosphodiesterase (Homo sapiens (Human)) | BDBM236644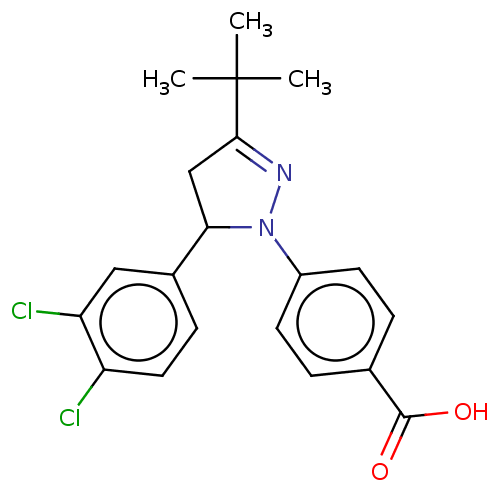 (US9388139, 13) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 5.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
German University; Southern Research Institute US Patent | Assay Description PDE activity was measured using the IMAP fluorescence polarization assay (Molecular Devices) in which binding of hydrolyzed cyclic nucleotide substra... | US Patent US9388139 (2016) BindingDB Entry DOI: 10.7270/Q2GF0SFS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| cGMP-specific 3',5'-cyclic phosphodiesterase (Homo sapiens (Human)) | BDBM236635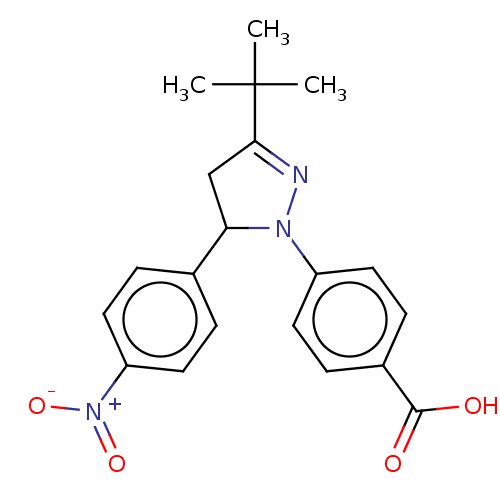 (US9388139, 2) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 5.70E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
German University; Southern Research Institute US Patent | Assay Description PDE activity was measured using the IMAP fluorescence polarization assay (Molecular Devices) in which binding of hydrolyzed cyclic nucleotide substra... | US Patent US9388139 (2016) BindingDB Entry DOI: 10.7270/Q2GF0SFS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| cGMP-specific 3',5'-cyclic phosphodiesterase (Homo sapiens (Human)) | BDBM236653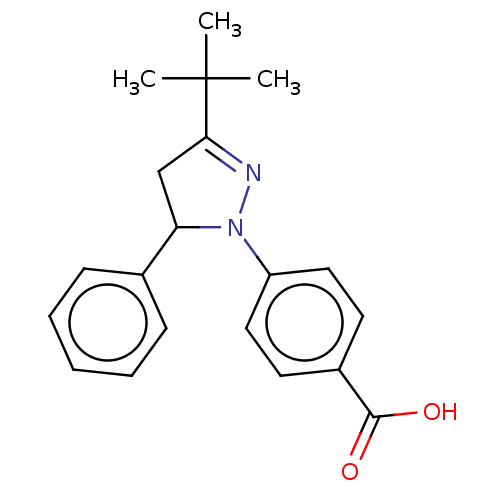 (US9388139, 22) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 6.22E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
German University; Southern Research Institute US Patent | Assay Description PDE activity was measured using the IMAP fluorescence polarization assay (Molecular Devices) in which binding of hydrolyzed cyclic nucleotide substra... | US Patent US9388139 (2016) BindingDB Entry DOI: 10.7270/Q2GF0SFS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| cGMP-specific 3',5'-cyclic phosphodiesterase (Homo sapiens (Human)) | BDBM236642 (US9388139, 10) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 6.90E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
German University; Southern Research Institute US Patent | Assay Description PDE activity was measured using the IMAP fluorescence polarization assay (Molecular Devices) in which binding of hydrolyzed cyclic nucleotide substra... | US Patent US9388139 (2016) BindingDB Entry DOI: 10.7270/Q2GF0SFS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| cGMP-specific 3',5'-cyclic phosphodiesterase (Homo sapiens (Human)) | BDBM236645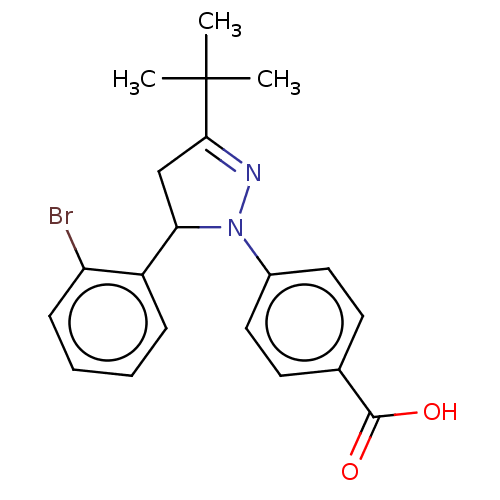 (US9388139, 14) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 6.90E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
German University; Southern Research Institute US Patent | Assay Description PDE activity was measured using the IMAP fluorescence polarization assay (Molecular Devices) in which binding of hydrolyzed cyclic nucleotide substra... | US Patent US9388139 (2016) BindingDB Entry DOI: 10.7270/Q2GF0SFS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| cGMP-specific 3',5'-cyclic phosphodiesterase (Homo sapiens (Human)) | BDBM236657 (US9388139, 26) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 7.20E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
German University; Southern Research Institute US Patent | Assay Description PDE activity was measured using the IMAP fluorescence polarization assay (Molecular Devices) in which binding of hydrolyzed cyclic nucleotide substra... | US Patent US9388139 (2016) BindingDB Entry DOI: 10.7270/Q2GF0SFS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| cGMP-specific 3',5'-cyclic phosphodiesterase (Homo sapiens (Human)) | BDBM236655 (US9388139, 24) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 7.70E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
German University; Southern Research Institute US Patent | Assay Description PDE activity was measured using the IMAP fluorescence polarization assay (Molecular Devices) in which binding of hydrolyzed cyclic nucleotide substra... | US Patent US9388139 (2016) BindingDB Entry DOI: 10.7270/Q2GF0SFS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| cGMP-specific 3',5'-cyclic phosphodiesterase (Homo sapiens (Human)) | BDBM236654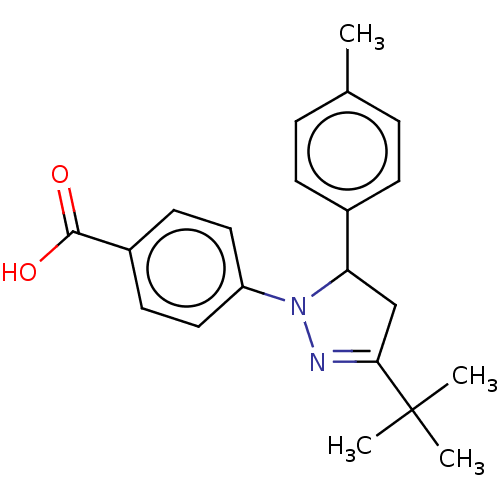 (US9388139, 23) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 8.40E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
German University; Southern Research Institute US Patent | Assay Description PDE activity was measured using the IMAP fluorescence polarization assay (Molecular Devices) in which binding of hydrolyzed cyclic nucleotide substra... | US Patent US9388139 (2016) BindingDB Entry DOI: 10.7270/Q2GF0SFS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| cGMP-specific 3',5'-cyclic phosphodiesterase (Homo sapiens (Human)) | BDBM236652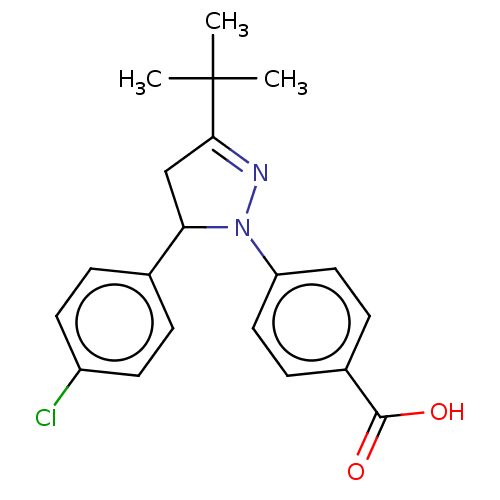 (US9388139, 21) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 8.70E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
German University; Southern Research Institute US Patent | Assay Description PDE activity was measured using the IMAP fluorescence polarization assay (Molecular Devices) in which binding of hydrolyzed cyclic nucleotide substra... | US Patent US9388139 (2016) BindingDB Entry DOI: 10.7270/Q2GF0SFS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| cGMP-specific 3',5'-cyclic phosphodiesterase (Homo sapiens (Human)) | BDBM236639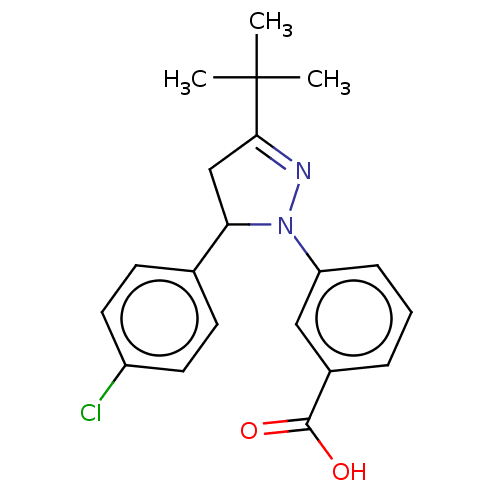 (US9388139, 6) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 9.90E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
German University; Southern Research Institute US Patent | Assay Description PDE activity was measured using the IMAP fluorescence polarization assay (Molecular Devices) in which binding of hydrolyzed cyclic nucleotide substra... | US Patent US9388139 (2016) BindingDB Entry DOI: 10.7270/Q2GF0SFS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin G/H synthase 1 (Homo sapiens (Human)) | BDBM11639 (4-[5-(4-methylphenyl)-3-(trifluoromethyl)-1H-pyraz...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | US Patent | n/a | n/a | 2.30E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
German University; Southern Research Institute US Patent | Assay Description The activities of COX-1 and COX-2 were measured after the addition of arachidonic acid and incubation at 25° C. for 5 min by absorbance at 590 nm as ... | US Patent US9388139 (2016) BindingDB Entry DOI: 10.7270/Q2GF0SFS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| cGMP-specific 3',5'-cyclic phosphodiesterase (Homo sapiens (Human)) | BDBM236637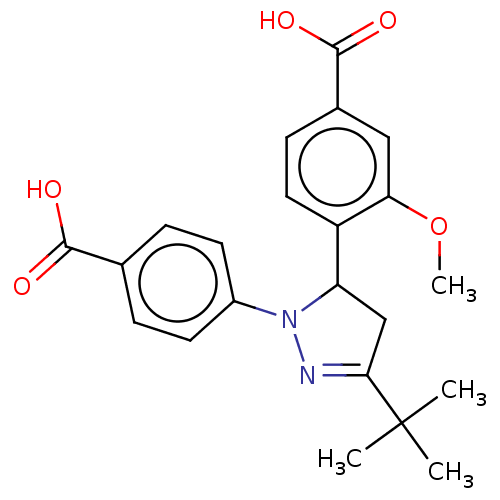 (US9388139, 4) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 2.35E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
German University; Southern Research Institute US Patent | Assay Description PDE activity was measured using the IMAP fluorescence polarization assay (Molecular Devices) in which binding of hydrolyzed cyclic nucleotide substra... | US Patent US9388139 (2016) BindingDB Entry DOI: 10.7270/Q2GF0SFS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| cGMP-specific 3',5'-cyclic phosphodiesterase (Homo sapiens (Human)) | BDBM11639 (4-[5-(4-methylphenyl)-3-(trifluoromethyl)-1H-pyraz...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | US Patent | n/a | n/a | 3.60E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
German University; Southern Research Institute US Patent | Assay Description PDE activity was measured using the IMAP fluorescence polarization assay (Molecular Devices) in which binding of hydrolyzed cyclic nucleotide substra... | US Patent US9388139 (2016) BindingDB Entry DOI: 10.7270/Q2GF0SFS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| cGMP-inhibited 3',5'-cyclic phosphodiesterase 3A (Homo sapiens (Human)) | BDBM236643 (US9388139, 12) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | >5.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
German University; Southern Research Institute US Patent | Assay Description PDE activity was measured using the IMAP fluorescence polarization assay (Molecular Devices) in which binding of hydrolyzed cyclic nucleotide substra... | US Patent US9388139 (2016) BindingDB Entry DOI: 10.7270/Q2GF0SFS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dual specificity calcium/calmodulin-dependent 3',5'-cyclic nucleotide phosphodiesterase 1A (Homo sapiens (Human)) | BDBM236643 (US9388139, 12) | KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | >5.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
German University; Southern Research Institute US Patent | Assay Description PDE activity was measured using the IMAP fluorescence polarization assay (Molecular Devices) in which binding of hydrolyzed cyclic nucleotide substra... | US Patent US9388139 (2016) BindingDB Entry DOI: 10.7270/Q2GF0SFS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| cGMP-inhibited 3',5'-cyclic phosphodiesterase 3B (Homo sapiens (Human)) | BDBM236643 (US9388139, 12) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | >5.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
German University; Southern Research Institute US Patent | Assay Description PDE activity was measured using the IMAP fluorescence polarization assay (Molecular Devices) in which binding of hydrolyzed cyclic nucleotide substra... | US Patent US9388139 (2016) BindingDB Entry DOI: 10.7270/Q2GF0SFS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Isoform PDE2A1 of cGMP-dependent 3',5'-cyclic phosphodiesterase (PDE2A1) (Homo sapiens (Human)) | BDBM236643 (US9388139, 12) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | >5.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
German University; Southern Research Institute US Patent | Assay Description PDE activity was measured using the IMAP fluorescence polarization assay (Molecular Devices) in which binding of hydrolyzed cyclic nucleotide substra... | US Patent US9388139 (2016) BindingDB Entry DOI: 10.7270/Q2GF0SFS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| High affinity cGMP-specific 3',5'-cyclic phosphodiesterase 9A (Homo sapiens (Human)) | BDBM236643 (US9388139, 12) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | >5.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
German University; Southern Research Institute US Patent | Assay Description PDE activity was measured using the IMAP fluorescence polarization assay (Molecular Devices) in which binding of hydrolyzed cyclic nucleotide substra... | US Patent US9388139 (2016) BindingDB Entry DOI: 10.7270/Q2GF0SFS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Isoform 4 of Dual 3',5'-cyclic-AMP and -GMP phosphodiesterase 11A (PDE11A1) (Homo sapiens (Human)) | BDBM236643 (US9388139, 12) | UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | >5.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
German University; Southern Research Institute US Patent | Assay Description PDE activity was measured using the IMAP fluorescence polarization assay (Molecular Devices) in which binding of hydrolyzed cyclic nucleotide substra... | US Patent US9388139 (2016) BindingDB Entry DOI: 10.7270/Q2GF0SFS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Isoform 4 of Dual 3',5'-cyclic-AMP and -GMP phosphodiesterase 11A (PDE11A1) (Homo sapiens (Human)) | BDBM236643 (US9388139, 12) | UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | >5.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
German University; Southern Research Institute US Patent | Assay Description PDE activity was measured using the IMAP fluorescence polarization assay (Molecular Devices) in which binding of hydrolyzed cyclic nucleotide substra... | US Patent US9388139 (2016) BindingDB Entry DOI: 10.7270/Q2GF0SFS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dual specificity calcium/calmodulin-dependent 3',5'-cyclic nucleotide phosphodiesterase 1A (Homo sapiens (Human)) | BDBM236643 (US9388139, 12) | KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | >5.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
German University; Southern Research Institute US Patent | Assay Description PDE activity was measured using the IMAP fluorescence polarization assay (Molecular Devices) in which binding of hydrolyzed cyclic nucleotide substra... | US Patent US9388139 (2016) BindingDB Entry DOI: 10.7270/Q2GF0SFS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Isoform PDE2A1 of cGMP-dependent 3',5'-cyclic phosphodiesterase (PDE2A1) (Homo sapiens (Human)) | BDBM236643 (US9388139, 12) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | >5.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
German University; Southern Research Institute US Patent | Assay Description PDE activity was measured using the IMAP fluorescence polarization assay (Molecular Devices) in which binding of hydrolyzed cyclic nucleotide substra... | US Patent US9388139 (2016) BindingDB Entry DOI: 10.7270/Q2GF0SFS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| cGMP-inhibited 3',5'-cyclic phosphodiesterase 3A (Homo sapiens (Human)) | BDBM236643 (US9388139, 12) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | >5.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
German University; Southern Research Institute US Patent | Assay Description PDE activity was measured using the IMAP fluorescence polarization assay (Molecular Devices) in which binding of hydrolyzed cyclic nucleotide substra... | US Patent US9388139 (2016) BindingDB Entry DOI: 10.7270/Q2GF0SFS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| cAMP and cAMP-inhibited cGMP 3',5'-cyclic phosphodiesterase 10A (Homo sapiens (Human)) | BDBM236643 (US9388139, 12) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | >5.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
German University; Southern Research Institute US Patent | Assay Description PDE activity was measured using the IMAP fluorescence polarization assay (Molecular Devices) in which binding of hydrolyzed cyclic nucleotide substra... | US Patent US9388139 (2016) BindingDB Entry DOI: 10.7270/Q2GF0SFS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| cGMP-inhibited 3',5'-cyclic phosphodiesterase 3B (Homo sapiens (Human)) | BDBM236643 (US9388139, 12) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | >5.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
German University; Southern Research Institute US Patent | Assay Description PDE activity was measured using the IMAP fluorescence polarization assay (Molecular Devices) in which binding of hydrolyzed cyclic nucleotide substra... | US Patent US9388139 (2016) BindingDB Entry DOI: 10.7270/Q2GF0SFS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| cAMP and cAMP-inhibited cGMP 3',5'-cyclic phosphodiesterase 10A (Homo sapiens (Human)) | BDBM236643 (US9388139, 12) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | >5.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
German University; Southern Research Institute US Patent | Assay Description PDE activity was measured using the IMAP fluorescence polarization assay (Molecular Devices) in which binding of hydrolyzed cyclic nucleotide substra... | US Patent US9388139 (2016) BindingDB Entry DOI: 10.7270/Q2GF0SFS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin G/H synthase 1 (Homo sapiens (Human)) | BDBM236638 (US9388139, 5) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
German University; Southern Research Institute US Patent | Assay Description The activities of COX-1 and COX-2 were measured after the addition of arachidonic acid and incubation at 25° C. for 5 min by absorbance at 590 nm as ... | US Patent US9388139 (2016) BindingDB Entry DOI: 10.7270/Q2GF0SFS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin G/H synthase 2 (Homo sapiens (Human)) | BDBM236648 (US9388139, 17) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
German University; Southern Research Institute US Patent | Assay Description The activities of COX-1 and COX-2 were measured after the addition of arachidonic acid and incubation at 25° C. for 5 min by absorbance at 590 nm as ... | US Patent US9388139 (2016) BindingDB Entry DOI: 10.7270/Q2GF0SFS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin G/H synthase 1 (Homo sapiens (Human)) | BDBM236647 (US9388139, 16) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
German University; Southern Research Institute US Patent | Assay Description The activities of COX-1 and COX-2 were measured after the addition of arachidonic acid and incubation at 25° C. for 5 min by absorbance at 590 nm as ... | US Patent US9388139 (2016) BindingDB Entry DOI: 10.7270/Q2GF0SFS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin G/H synthase 2 (Homo sapiens (Human)) | BDBM236639 (US9388139, 6) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
German University; Southern Research Institute US Patent | Assay Description The activities of COX-1 and COX-2 were measured after the addition of arachidonic acid and incubation at 25° C. for 5 min by absorbance at 590 nm as ... | US Patent US9388139 (2016) BindingDB Entry DOI: 10.7270/Q2GF0SFS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin G/H synthase 2 (Homo sapiens (Human)) | BDBM236642 (US9388139, 10) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
German University; Southern Research Institute US Patent | Assay Description The activities of COX-1 and COX-2 were measured after the addition of arachidonic acid and incubation at 25° C. for 5 min by absorbance at 590 nm as ... | US Patent US9388139 (2016) BindingDB Entry DOI: 10.7270/Q2GF0SFS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin G/H synthase 2 (Homo sapiens (Human)) | BDBM236647 (US9388139, 16) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
German University; Southern Research Institute US Patent | Assay Description The activities of COX-1 and COX-2 were measured after the addition of arachidonic acid and incubation at 25° C. for 5 min by absorbance at 590 nm as ... | US Patent US9388139 (2016) BindingDB Entry DOI: 10.7270/Q2GF0SFS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin G/H synthase 2 (Homo sapiens (Human)) | BDBM236634 (US9388139, 1) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
German University; Southern Research Institute US Patent | Assay Description The activities of COX-1 and COX-2 were measured after the addition of arachidonic acid and incubation at 25° C. for 5 min by absorbance at 590 nm as ... | US Patent US9388139 (2016) BindingDB Entry DOI: 10.7270/Q2GF0SFS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin G/H synthase 2 (Homo sapiens (Human)) | BDBM236636 (US9388139, 3) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
German University; Southern Research Institute US Patent | Assay Description The activities of COX-1 and COX-2 were measured after the addition of arachidonic acid and incubation at 25° C. for 5 min by absorbance at 590 nm as ... | US Patent US9388139 (2016) BindingDB Entry DOI: 10.7270/Q2GF0SFS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin G/H synthase 2 (Homo sapiens (Human)) | BDBM236638 (US9388139, 5) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
German University; Southern Research Institute US Patent | Assay Description The activities of COX-1 and COX-2 were measured after the addition of arachidonic acid and incubation at 25° C. for 5 min by absorbance at 590 nm as ... | US Patent US9388139 (2016) BindingDB Entry DOI: 10.7270/Q2GF0SFS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin G/H synthase 2 (Homo sapiens (Human)) | BDBM236649 (US9388139, 18) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
German University; Southern Research Institute US Patent | Assay Description The activities of COX-1 and COX-2 were measured after the addition of arachidonic acid and incubation at 25° C. for 5 min by absorbance at 590 nm as ... | US Patent US9388139 (2016) BindingDB Entry DOI: 10.7270/Q2GF0SFS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin G/H synthase 2 (Homo sapiens (Human)) | BDBM236656 (US9388139, 25) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
German University; Southern Research Institute US Patent | Assay Description The activities of COX-1 and COX-2 were measured after the addition of arachidonic acid and incubation at 25° C. for 5 min by absorbance at 590 nm as ... | US Patent US9388139 (2016) BindingDB Entry DOI: 10.7270/Q2GF0SFS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin G/H synthase 1 (Homo sapiens (Human)) | BDBM236634 (US9388139, 1) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
German University; Southern Research Institute US Patent | Assay Description The activities of COX-1 and COX-2 were measured after the addition of arachidonic acid and incubation at 25° C. for 5 min by absorbance at 590 nm as ... | US Patent US9388139 (2016) BindingDB Entry DOI: 10.7270/Q2GF0SFS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin G/H synthase 1 (Homo sapiens (Human)) | BDBM236639 (US9388139, 6) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
German University; Southern Research Institute US Patent | Assay Description The activities of COX-1 and COX-2 were measured after the addition of arachidonic acid and incubation at 25° C. for 5 min by absorbance at 590 nm as ... | US Patent US9388139 (2016) BindingDB Entry DOI: 10.7270/Q2GF0SFS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin G/H synthase 1 (Homo sapiens (Human)) | BDBM236645 (US9388139, 14) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
German University; Southern Research Institute US Patent | Assay Description The activities of COX-1 and COX-2 were measured after the addition of arachidonic acid and incubation at 25° C. for 5 min by absorbance at 590 nm as ... | US Patent US9388139 (2016) BindingDB Entry DOI: 10.7270/Q2GF0SFS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin G/H synthase 1 (Homo sapiens (Human)) | BDBM236657 (US9388139, 26) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
German University; Southern Research Institute US Patent | Assay Description The activities of COX-1 and COX-2 were measured after the addition of arachidonic acid and incubation at 25° C. for 5 min by absorbance at 590 nm as ... | US Patent US9388139 (2016) BindingDB Entry DOI: 10.7270/Q2GF0SFS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin G/H synthase 2 (Homo sapiens (Human)) | BDBM236637 (US9388139, 4) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
German University; Southern Research Institute US Patent | Assay Description The activities of COX-1 and COX-2 were measured after the addition of arachidonic acid and incubation at 25° C. for 5 min by absorbance at 590 nm as ... | US Patent US9388139 (2016) BindingDB Entry DOI: 10.7270/Q2GF0SFS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin G/H synthase 2 (Homo sapiens (Human)) | BDBM54632 (4-[3-(4-methoxyphenyl)-5-phenyl-3,4-dihydropyrazol...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase PC cid PC sid UniChem Similars | US Patent | n/a | n/a | >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
German University; Southern Research Institute US Patent | Assay Description The activities of COX-1 and COX-2 were measured after the addition of arachidonic acid and incubation at 25° C. for 5 min by absorbance at 590 nm as ... | US Patent US9388139 (2016) BindingDB Entry DOI: 10.7270/Q2GF0SFS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin G/H synthase 2 (Homo sapiens (Human)) | BDBM43220 (4-[3-(4-methoxyphenyl)-5-phenyl-3,4-dihydropyrazol...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem Similars | US Patent | n/a | n/a | >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
German University; Southern Research Institute US Patent | Assay Description The activities of COX-1 and COX-2 were measured after the addition of arachidonic acid and incubation at 25° C. for 5 min by absorbance at 590 nm as ... | US Patent US9388139 (2016) BindingDB Entry DOI: 10.7270/Q2GF0SFS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin G/H synthase 2 (Homo sapiens (Human)) | BDBM236644 (US9388139, 13) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
German University; Southern Research Institute US Patent | Assay Description The activities of COX-1 and COX-2 were measured after the addition of arachidonic acid and incubation at 25° C. for 5 min by absorbance at 590 nm as ... | US Patent US9388139 (2016) BindingDB Entry DOI: 10.7270/Q2GF0SFS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin G/H synthase 2 (Homo sapiens (Human)) | BDBM236646 (US9388139, 15) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
German University; Southern Research Institute US Patent | Assay Description The activities of COX-1 and COX-2 were measured after the addition of arachidonic acid and incubation at 25° C. for 5 min by absorbance at 590 nm as ... | US Patent US9388139 (2016) BindingDB Entry DOI: 10.7270/Q2GF0SFS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin G/H synthase 2 (Homo sapiens (Human)) | BDBM236650 (US9388139, 19) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
German University; Southern Research Institute US Patent | Assay Description The activities of COX-1 and COX-2 were measured after the addition of arachidonic acid and incubation at 25° C. for 5 min by absorbance at 590 nm as ... | US Patent US9388139 (2016) BindingDB Entry DOI: 10.7270/Q2GF0SFS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin G/H synthase 2 (Homo sapiens (Human)) | BDBM236651 (US9388139, 20) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
German University; Southern Research Institute US Patent | Assay Description The activities of COX-1 and COX-2 were measured after the addition of arachidonic acid and incubation at 25° C. for 5 min by absorbance at 590 nm as ... | US Patent US9388139 (2016) BindingDB Entry DOI: 10.7270/Q2GF0SFS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin G/H synthase 2 (Homo sapiens (Human)) | BDBM236657 (US9388139, 26) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
German University; Southern Research Institute US Patent | Assay Description The activities of COX-1 and COX-2 were measured after the addition of arachidonic acid and incubation at 25° C. for 5 min by absorbance at 590 nm as ... | US Patent US9388139 (2016) BindingDB Entry DOI: 10.7270/Q2GF0SFS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin G/H synthase 1 (Homo sapiens (Human)) | BDBM236644 (US9388139, 13) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
German University; Southern Research Institute US Patent | Assay Description The activities of COX-1 and COX-2 were measured after the addition of arachidonic acid and incubation at 25° C. for 5 min by absorbance at 590 nm as ... | US Patent US9388139 (2016) BindingDB Entry DOI: 10.7270/Q2GF0SFS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin G/H synthase 2 (Homo sapiens (Human)) | BDBM236653 (US9388139, 22) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
German University; Southern Research Institute US Patent | Assay Description The activities of COX-1 and COX-2 were measured after the addition of arachidonic acid and incubation at 25° C. for 5 min by absorbance at 590 nm as ... | US Patent US9388139 (2016) BindingDB Entry DOI: 10.7270/Q2GF0SFS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin G/H synthase 2 (Homo sapiens (Human)) | BDBM236655 (US9388139, 24) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
German University; Southern Research Institute US Patent | Assay Description The activities of COX-1 and COX-2 were measured after the addition of arachidonic acid and incubation at 25° C. for 5 min by absorbance at 590 nm as ... | US Patent US9388139 (2016) BindingDB Entry DOI: 10.7270/Q2GF0SFS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin G/H synthase 1 (Homo sapiens (Human)) | BDBM54632 (4-[3-(4-methoxyphenyl)-5-phenyl-3,4-dihydropyrazol...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase PC cid PC sid UniChem Similars | US Patent | n/a | n/a | >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
German University; Southern Research Institute US Patent | Assay Description The activities of COX-1 and COX-2 were measured after the addition of arachidonic acid and incubation at 25° C. for 5 min by absorbance at 590 nm as ... | US Patent US9388139 (2016) BindingDB Entry DOI: 10.7270/Q2GF0SFS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin G/H synthase 1 (Homo sapiens (Human)) | BDBM236643 (US9388139, 12) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
German University; Southern Research Institute US Patent | Assay Description The activities of COX-1 and COX-2 were measured after the addition of arachidonic acid and incubation at 25° C. for 5 min by absorbance at 590 nm as ... | US Patent US9388139 (2016) BindingDB Entry DOI: 10.7270/Q2GF0SFS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin G/H synthase 1 (Homo sapiens (Human)) | BDBM236646 (US9388139, 15) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
German University; Southern Research Institute US Patent | Assay Description The activities of COX-1 and COX-2 were measured after the addition of arachidonic acid and incubation at 25° C. for 5 min by absorbance at 590 nm as ... | US Patent US9388139 (2016) BindingDB Entry DOI: 10.7270/Q2GF0SFS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin G/H synthase 1 (Homo sapiens (Human)) | BDBM236651 (US9388139, 20) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
German University; Southern Research Institute US Patent | Assay Description The activities of COX-1 and COX-2 were measured after the addition of arachidonic acid and incubation at 25° C. for 5 min by absorbance at 590 nm as ... | US Patent US9388139 (2016) BindingDB Entry DOI: 10.7270/Q2GF0SFS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin G/H synthase 1 (Homo sapiens (Human)) | BDBM236652 (US9388139, 21) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
German University; Southern Research Institute US Patent | Assay Description The activities of COX-1 and COX-2 were measured after the addition of arachidonic acid and incubation at 25° C. for 5 min by absorbance at 590 nm as ... | US Patent US9388139 (2016) BindingDB Entry DOI: 10.7270/Q2GF0SFS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin G/H synthase 1 (Homo sapiens (Human)) | BDBM236654 (US9388139, 23) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
German University; Southern Research Institute US Patent | Assay Description The activities of COX-1 and COX-2 were measured after the addition of arachidonic acid and incubation at 25° C. for 5 min by absorbance at 590 nm as ... | US Patent US9388139 (2016) BindingDB Entry DOI: 10.7270/Q2GF0SFS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin G/H synthase 1 (Homo sapiens (Human)) | BDBM236656 (US9388139, 25) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
German University; Southern Research Institute US Patent | Assay Description The activities of COX-1 and COX-2 were measured after the addition of arachidonic acid and incubation at 25° C. for 5 min by absorbance at 590 nm as ... | US Patent US9388139 (2016) BindingDB Entry DOI: 10.7270/Q2GF0SFS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin G/H synthase 2 (Homo sapiens (Human)) | BDBM236635 (US9388139, 2) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
German University; Southern Research Institute US Patent | Assay Description The activities of COX-1 and COX-2 were measured after the addition of arachidonic acid and incubation at 25° C. for 5 min by absorbance at 590 nm as ... | US Patent US9388139 (2016) BindingDB Entry DOI: 10.7270/Q2GF0SFS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin G/H synthase 2 (Homo sapiens (Human)) | BDBM236640 (US9388139, 8) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
German University; Southern Research Institute US Patent | Assay Description The activities of COX-1 and COX-2 were measured after the addition of arachidonic acid and incubation at 25° C. for 5 min by absorbance at 590 nm as ... | US Patent US9388139 (2016) BindingDB Entry DOI: 10.7270/Q2GF0SFS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin G/H synthase 2 (Homo sapiens (Human)) | BDBM236652 (US9388139, 21) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
German University; Southern Research Institute US Patent | Assay Description The activities of COX-1 and COX-2 were measured after the addition of arachidonic acid and incubation at 25° C. for 5 min by absorbance at 590 nm as ... | US Patent US9388139 (2016) BindingDB Entry DOI: 10.7270/Q2GF0SFS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin G/H synthase 2 (Homo sapiens (Human)) | BDBM236658 (US9388139, 27) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
German University; Southern Research Institute US Patent | Assay Description The activities of COX-1 and COX-2 were measured after the addition of arachidonic acid and incubation at 25° C. for 5 min by absorbance at 590 nm as ... | US Patent US9388139 (2016) BindingDB Entry DOI: 10.7270/Q2GF0SFS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin G/H synthase 1 (Homo sapiens (Human)) | BDBM236648 (US9388139, 17) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
German University; Southern Research Institute US Patent | Assay Description The activities of COX-1 and COX-2 were measured after the addition of arachidonic acid and incubation at 25° C. for 5 min by absorbance at 590 nm as ... | US Patent US9388139 (2016) BindingDB Entry DOI: 10.7270/Q2GF0SFS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin G/H synthase 1 (Homo sapiens (Human)) | BDBM236650 (US9388139, 19) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
German University; Southern Research Institute US Patent | Assay Description The activities of COX-1 and COX-2 were measured after the addition of arachidonic acid and incubation at 25° C. for 5 min by absorbance at 590 nm as ... | US Patent US9388139 (2016) BindingDB Entry DOI: 10.7270/Q2GF0SFS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin G/H synthase 2 (Homo sapiens (Human)) | BDBM236643 (US9388139, 12) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
German University; Southern Research Institute US Patent | Assay Description The activities of COX-1 and COX-2 were measured after the addition of arachidonic acid and incubation at 25° C. for 5 min by absorbance at 590 nm as ... | US Patent US9388139 (2016) BindingDB Entry DOI: 10.7270/Q2GF0SFS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin G/H synthase 2 (Homo sapiens (Human)) | BDBM236654 (US9388139, 23) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
German University; Southern Research Institute US Patent | Assay Description The activities of COX-1 and COX-2 were measured after the addition of arachidonic acid and incubation at 25° C. for 5 min by absorbance at 590 nm as ... | US Patent US9388139 (2016) BindingDB Entry DOI: 10.7270/Q2GF0SFS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin G/H synthase 2 (Homo sapiens (Human)) | BDBM236641 (US9388139, 9) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
German University; Southern Research Institute US Patent | Assay Description The activities of COX-1 and COX-2 were measured after the addition of arachidonic acid and incubation at 25° C. for 5 min by absorbance at 590 nm as ... | US Patent US9388139 (2016) BindingDB Entry DOI: 10.7270/Q2GF0SFS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin G/H synthase 1 (Homo sapiens (Human)) | BDBM236635 (US9388139, 2) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
German University; Southern Research Institute US Patent | Assay Description The activities of COX-1 and COX-2 were measured after the addition of arachidonic acid and incubation at 25° C. for 5 min by absorbance at 590 nm as ... | US Patent US9388139 (2016) BindingDB Entry DOI: 10.7270/Q2GF0SFS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin G/H synthase 1 (Homo sapiens (Human)) | BDBM236636 (US9388139, 3) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
German University; Southern Research Institute US Patent | Assay Description The activities of COX-1 and COX-2 were measured after the addition of arachidonic acid and incubation at 25° C. for 5 min by absorbance at 590 nm as ... | US Patent US9388139 (2016) BindingDB Entry DOI: 10.7270/Q2GF0SFS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin G/H synthase 1 (Homo sapiens (Human)) | BDBM236637 (US9388139, 4) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
German University; Southern Research Institute US Patent | Assay Description The activities of COX-1 and COX-2 were measured after the addition of arachidonic acid and incubation at 25° C. for 5 min by absorbance at 590 nm as ... | US Patent US9388139 (2016) BindingDB Entry DOI: 10.7270/Q2GF0SFS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin G/H synthase 1 (Homo sapiens (Human)) | BDBM236640 (US9388139, 8) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
German University; Southern Research Institute US Patent | Assay Description The activities of COX-1 and COX-2 were measured after the addition of arachidonic acid and incubation at 25° C. for 5 min by absorbance at 590 nm as ... | US Patent US9388139 (2016) BindingDB Entry DOI: 10.7270/Q2GF0SFS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin G/H synthase 1 (Homo sapiens (Human)) | BDBM236641 (US9388139, 9) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
German University; Southern Research Institute US Patent | Assay Description The activities of COX-1 and COX-2 were measured after the addition of arachidonic acid and incubation at 25° C. for 5 min by absorbance at 590 nm as ... | US Patent US9388139 (2016) BindingDB Entry DOI: 10.7270/Q2GF0SFS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin G/H synthase 1 (Homo sapiens (Human)) | BDBM43220 (4-[3-(4-methoxyphenyl)-5-phenyl-3,4-dihydropyrazol...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem Similars | US Patent | n/a | n/a | >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
German University; Southern Research Institute US Patent | Assay Description The activities of COX-1 and COX-2 were measured after the addition of arachidonic acid and incubation at 25° C. for 5 min by absorbance at 590 nm as ... | US Patent US9388139 (2016) BindingDB Entry DOI: 10.7270/Q2GF0SFS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin G/H synthase 1 (Homo sapiens (Human)) | BDBM236649 (US9388139, 18) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
German University; Southern Research Institute US Patent | Assay Description The activities of COX-1 and COX-2 were measured after the addition of arachidonic acid and incubation at 25° C. for 5 min by absorbance at 590 nm as ... | US Patent US9388139 (2016) BindingDB Entry DOI: 10.7270/Q2GF0SFS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin G/H synthase 1 (Homo sapiens (Human)) | BDBM236653 (US9388139, 22) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
German University; Southern Research Institute US Patent | Assay Description The activities of COX-1 and COX-2 were measured after the addition of arachidonic acid and incubation at 25° C. for 5 min by absorbance at 590 nm as ... | US Patent US9388139 (2016) BindingDB Entry DOI: 10.7270/Q2GF0SFS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin G/H synthase 1 (Homo sapiens (Human)) | BDBM236655 (US9388139, 24) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
German University; Southern Research Institute US Patent | Assay Description The activities of COX-1 and COX-2 were measured after the addition of arachidonic acid and incubation at 25° C. for 5 min by absorbance at 590 nm as ... | US Patent US9388139 (2016) BindingDB Entry DOI: 10.7270/Q2GF0SFS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin G/H synthase 1 (Homo sapiens (Human)) | BDBM236658 (US9388139, 27) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
German University; Southern Research Institute US Patent | Assay Description The activities of COX-1 and COX-2 were measured after the addition of arachidonic acid and incubation at 25° C. for 5 min by absorbance at 590 nm as ... | US Patent US9388139 (2016) BindingDB Entry DOI: 10.7270/Q2GF0SFS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin G/H synthase 2 (Homo sapiens (Human)) | BDBM236645 (US9388139, 14) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
German University; Southern Research Institute US Patent | Assay Description The activities of COX-1 and COX-2 were measured after the addition of arachidonic acid and incubation at 25° C. for 5 min by absorbance at 590 nm as ... | US Patent US9388139 (2016) BindingDB Entry DOI: 10.7270/Q2GF0SFS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin G/H synthase 1 (Homo sapiens (Human)) | BDBM236642 (US9388139, 10) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
German University; Southern Research Institute US Patent | Assay Description The activities of COX-1 and COX-2 were measured after the addition of arachidonic acid and incubation at 25° C. for 5 min by absorbance at 590 nm as ... | US Patent US9388139 (2016) BindingDB Entry DOI: 10.7270/Q2GF0SFS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||