 Found 63 hits of Enzyme Inhibition Constant Data
Found 63 hits of Enzyme Inhibition Constant Data Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit beta isoform
(Homo sapiens (Human)) | BDBM255537

(US9499523, 11)Show SMILES C[C@H](Nc1nc(N)nc(N)c1C#N)c1nc2c(Cl)ccc(Cl)c2c(=O)n1-c1cnc(N)cn1 |r| Show InChI InChI=1S/C19H15Cl2N11O/c1-7(28-16-8(4-22)15(24)30-19(25)31-16)17-29-14-10(21)3-2-9(20)13(14)18(33)32(17)12-6-26-11(23)5-27-12/h2-3,5-7H,1H3,(H2,23,26)(H5,24,25,28,30,31)/t7-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | 1.80 | n/a | n/a | n/a | n/a | 7.4 | 25 |
GILEAD SCIENCES, INC.
US Patent
| Assay Description
The activities were measured using a time-resolved fluorescence resonance energy transfer (TR-FRET) assay. TR-FRET monitored the formation of 3,4,5-i... |
US Patent US9499523 (2016)
BindingDB Entry DOI: 10.7270/Q2S46QWR |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit delta isoform
(Homo sapiens (Human)) | BDBM255537

(US9499523, 11)Show SMILES C[C@H](Nc1nc(N)nc(N)c1C#N)c1nc2c(Cl)ccc(Cl)c2c(=O)n1-c1cnc(N)cn1 |r| Show InChI InChI=1S/C19H15Cl2N11O/c1-7(28-16-8(4-22)15(24)30-19(25)31-16)17-29-14-10(21)3-2-9(20)13(14)18(33)32(17)12-6-26-11(23)5-27-12/h2-3,5-7H,1H3,(H2,23,26)(H5,24,25,28,30,31)/t7-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | 1.90 | n/a | n/a | n/a | n/a | 7.4 | 25 |
GILEAD SCIENCES, INC.
US Patent
| Assay Description
The activities were measured using a time-resolved fluorescence resonance energy transfer (TR-FRET) assay. TR-FRET monitored the formation of 3,4,5-i... |
US Patent US9499523 (2016)
BindingDB Entry DOI: 10.7270/Q2S46QWR |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit delta isoform
(Homo sapiens (Human)) | BDBM255532

(US9499523, 6)Show SMILES C[C@H](Nc1nc(N)nc(N)c1C#N)c1nc2cccc(Cl)c2c(=O)n1-c1cnc(N)cn1 |r| Show InChI InChI=1S/C19H16ClN11O/c1-8(27-16-9(5-21)15(23)29-19(24)30-16)17-28-11-4-2-3-10(20)14(11)18(32)31(17)13-7-25-12(22)6-26-13/h2-4,6-8H,1H3,(H2,22,25)(H5,23,24,27,29,30)/t8-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | 2 | n/a | n/a | n/a | n/a | 7.4 | 25 |
GILEAD SCIENCES, INC.
US Patent
| Assay Description
The activities were measured using a time-resolved fluorescence resonance energy transfer (TR-FRET) assay. TR-FRET monitored the formation of 3,4,5-i... |
US Patent US9499523 (2016)
BindingDB Entry DOI: 10.7270/Q2S46QWR |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit beta isoform
(Homo sapiens (Human)) | BDBM255532

(US9499523, 6)Show SMILES C[C@H](Nc1nc(N)nc(N)c1C#N)c1nc2cccc(Cl)c2c(=O)n1-c1cnc(N)cn1 |r| Show InChI InChI=1S/C19H16ClN11O/c1-8(27-16-9(5-21)15(23)29-19(24)30-16)17-28-11-4-2-3-10(20)14(11)18(32)31(17)13-7-25-12(22)6-26-13/h2-4,6-8H,1H3,(H2,22,25)(H5,23,24,27,29,30)/t8-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | 2 | n/a | n/a | n/a | n/a | 7.4 | 25 |
GILEAD SCIENCES, INC.
US Patent
| Assay Description
The activities were measured using a time-resolved fluorescence resonance energy transfer (TR-FRET) assay. TR-FRET monitored the formation of 3,4,5-i... |
US Patent US9499523 (2016)
BindingDB Entry DOI: 10.7270/Q2S46QWR |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit delta isoform
(Homo sapiens (Human)) | BDBM255536
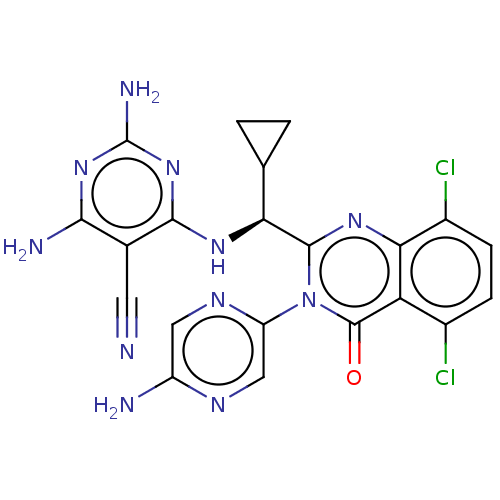
(US9499523, 10)Show SMILES Nc1cnc(cn1)-n1c(nc2c(Cl)ccc(Cl)c2c1=O)[C@@H](Nc1nc(N)nc(N)c1C#N)C1CC1 |r| Show InChI InChI=1S/C21H17Cl2N11O/c22-10-3-4-11(23)16-14(10)20(35)34(13-7-28-12(25)6-29-13)19(31-16)15(8-1-2-8)30-18-9(5-24)17(26)32-21(27)33-18/h3-4,6-8,15H,1-2H2,(H2,25,28)(H5,26,27,30,32,33)/t15-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | 2.40 | n/a | n/a | n/a | n/a | 7.4 | 25 |
GILEAD SCIENCES, INC.
US Patent
| Assay Description
The activities were measured using a time-resolved fluorescence resonance energy transfer (TR-FRET) assay. TR-FRET monitored the formation of 3,4,5-i... |
US Patent US9499523 (2016)
BindingDB Entry DOI: 10.7270/Q2S46QWR |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit delta isoform
(Homo sapiens (Human)) | BDBM255535
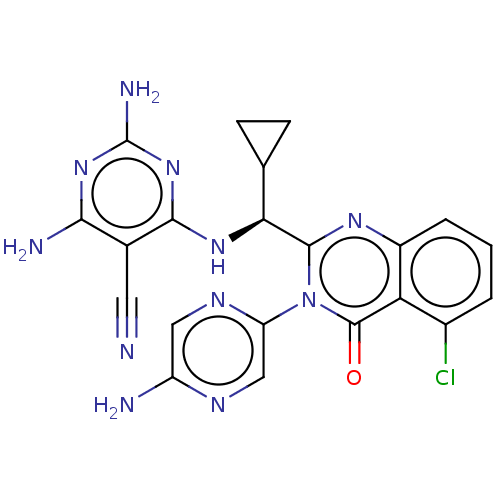
(US9499523, 9)Show SMILES Nc1cnc(cn1)-n1c(nc2cccc(Cl)c2c1=O)[C@@H](Nc1nc(N)nc(N)c1C#N)C1CC1 |r| Show InChI InChI=1S/C21H18ClN11O/c22-11-2-1-3-12-15(11)20(34)33(14-8-27-13(24)7-28-14)19(29-12)16(9-4-5-9)30-18-10(6-23)17(25)31-21(26)32-18/h1-3,7-9,16H,4-5H2,(H2,24,27)(H5,25,26,30,31,32)/t16-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | 2.70 | n/a | n/a | n/a | n/a | 7.4 | 25 |
GILEAD SCIENCES, INC.
US Patent
| Assay Description
The activities were measured using a time-resolved fluorescence resonance energy transfer (TR-FRET) assay. TR-FRET monitored the formation of 3,4,5-i... |
US Patent US9499523 (2016)
BindingDB Entry DOI: 10.7270/Q2S46QWR |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit delta isoform
(Homo sapiens (Human)) | BDBM255528

(US9499523, 2)Show SMILES C[C@H](Nc1nc(N)nc(N)c1C#N)c1nc2c(F)ccc(Cl)c2c(=O)n1-c1cnc(N)cn1 |r| Show InChI InChI=1S/C19H15ClFN11O/c1-7(28-16-8(4-22)15(24)30-19(25)31-16)17-29-14-10(21)3-2-9(20)13(14)18(33)32(17)12-6-26-11(23)5-27-12/h2-3,5-7H,1H3,(H2,23,26)(H5,24,25,28,30,31)/t7-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | 3.5 | n/a | n/a | n/a | n/a | 7.4 | 25 |
GILEAD SCIENCES, INC.
US Patent
| Assay Description
The activities were measured using a time-resolved fluorescence resonance energy transfer (TR-FRET) assay. TR-FRET monitored the formation of 3,4,5-i... |
US Patent US9499523 (2016)
BindingDB Entry DOI: 10.7270/Q2S46QWR |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit beta isoform
(Homo sapiens (Human)) | BDBM255536

(US9499523, 10)Show SMILES Nc1cnc(cn1)-n1c(nc2c(Cl)ccc(Cl)c2c1=O)[C@@H](Nc1nc(N)nc(N)c1C#N)C1CC1 |r| Show InChI InChI=1S/C21H17Cl2N11O/c22-10-3-4-11(23)16-14(10)20(35)34(13-7-28-12(25)6-29-13)19(31-16)15(8-1-2-8)30-18-9(5-24)17(26)32-21(27)33-18/h3-4,6-8,15H,1-2H2,(H2,25,28)(H5,26,27,30,32,33)/t15-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | 3.60 | n/a | n/a | n/a | n/a | 7.4 | 25 |
GILEAD SCIENCES, INC.
US Patent
| Assay Description
The activities were measured using a time-resolved fluorescence resonance energy transfer (TR-FRET) assay. TR-FRET monitored the formation of 3,4,5-i... |
US Patent US9499523 (2016)
BindingDB Entry DOI: 10.7270/Q2S46QWR |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit beta isoform
(Homo sapiens (Human)) | BDBM255535

(US9499523, 9)Show SMILES Nc1cnc(cn1)-n1c(nc2cccc(Cl)c2c1=O)[C@@H](Nc1nc(N)nc(N)c1C#N)C1CC1 |r| Show InChI InChI=1S/C21H18ClN11O/c22-11-2-1-3-12-15(11)20(34)33(14-8-27-13(24)7-28-14)19(29-12)16(9-4-5-9)30-18-10(6-23)17(25)31-21(26)32-18/h1-3,7-9,16H,4-5H2,(H2,24,27)(H5,25,26,30,31,32)/t16-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | 4.20 | n/a | n/a | n/a | n/a | 7.4 | 25 |
GILEAD SCIENCES, INC.
US Patent
| Assay Description
The activities were measured using a time-resolved fluorescence resonance energy transfer (TR-FRET) assay. TR-FRET monitored the formation of 3,4,5-i... |
US Patent US9499523 (2016)
BindingDB Entry DOI: 10.7270/Q2S46QWR |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit beta isoform
(Homo sapiens (Human)) | BDBM255528

(US9499523, 2)Show SMILES C[C@H](Nc1nc(N)nc(N)c1C#N)c1nc2c(F)ccc(Cl)c2c(=O)n1-c1cnc(N)cn1 |r| Show InChI InChI=1S/C19H15ClFN11O/c1-7(28-16-8(4-22)15(24)30-19(25)31-16)17-29-14-10(21)3-2-9(20)13(14)18(33)32(17)12-6-26-11(23)5-27-12/h2-3,5-7H,1H3,(H2,23,26)(H5,24,25,28,30,31)/t7-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | 4.40 | n/a | n/a | n/a | n/a | 7.4 | 25 |
GILEAD SCIENCES, INC.
US Patent
| Assay Description
The activities were measured using a time-resolved fluorescence resonance energy transfer (TR-FRET) assay. TR-FRET monitored the formation of 3,4,5-i... |
US Patent US9499523 (2016)
BindingDB Entry DOI: 10.7270/Q2S46QWR |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit beta isoform
(Homo sapiens (Human)) | BDBM255541
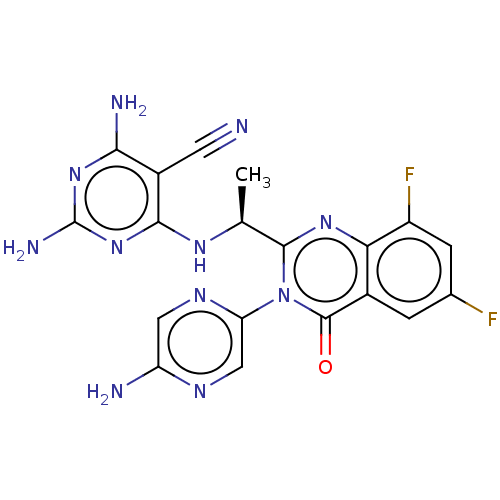
(US9499523, 15)Show SMILES C[C@H](Nc1nc(N)nc(N)c1C#N)c1nc2c(F)cc(F)cc2c(=O)n1-c1cnc(N)cn1 |r| Show InChI InChI=1S/C19H15F2N11O/c1-7(28-16-10(4-22)15(24)30-19(25)31-16)17-29-14-9(2-8(20)3-11(14)21)18(33)32(17)13-6-26-12(23)5-27-13/h2-3,5-7H,1H3,(H2,23,26)(H5,24,25,28,30,31)/t7-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | 9.80 | n/a | n/a | n/a | n/a | 7.4 | 25 |
GILEAD SCIENCES, INC.
US Patent
| Assay Description
The activities were measured using a time-resolved fluorescence resonance energy transfer (TR-FRET) assay. TR-FRET monitored the formation of 3,4,5-i... |
US Patent US9499523 (2016)
BindingDB Entry DOI: 10.7270/Q2S46QWR |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit delta isoform
(Homo sapiens (Human)) | BDBM255529
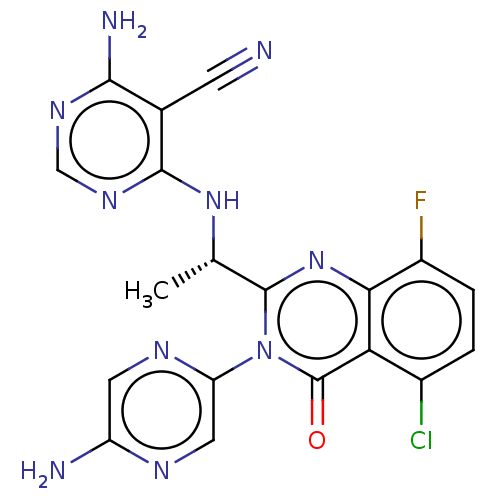
(US9499523, 3)Show SMILES C[C@H](Nc1ncnc(N)c1C#N)c1nc2c(F)ccc(Cl)c2c(=O)n1-c1cnc(N)cn1 |r| Show InChI InChI=1S/C19H14ClFN10O/c1-8(29-17-9(4-22)16(24)27-7-28-17)18-30-15-11(21)3-2-10(20)14(15)19(32)31(18)13-6-25-12(23)5-26-13/h2-3,5-8H,1H3,(H2,23,25)(H3,24,27,28,29)/t8-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | 11 | n/a | n/a | n/a | n/a | 7.4 | 25 |
GILEAD SCIENCES, INC.
US Patent
| Assay Description
The activities were measured using a time-resolved fluorescence resonance energy transfer (TR-FRET) assay. TR-FRET monitored the formation of 3,4,5-i... |
US Patent US9499523 (2016)
BindingDB Entry DOI: 10.7270/Q2S46QWR |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit beta isoform
(Homo sapiens (Human)) | BDBM255543

(US9499523, 17)Show SMILES C[C@H](Nc1nc(N)nc(N)c1C#N)c1nc2c(Cl)cc(F)cc2c(=O)n1-c1cnc(N)cn1 |r| Show InChI InChI=1S/C19H15ClFN11O/c1-7(28-16-10(4-22)15(24)30-19(25)31-16)17-29-14-9(2-8(21)3-11(14)20)18(33)32(17)13-6-26-12(23)5-27-13/h2-3,5-7H,1H3,(H2,23,26)(H5,24,25,28,30,31)/t7-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | 14 | n/a | n/a | n/a | n/a | 7.4 | 25 |
GILEAD SCIENCES, INC.
US Patent
| Assay Description
The activities were measured using a time-resolved fluorescence resonance energy transfer (TR-FRET) assay. TR-FRET monitored the formation of 3,4,5-i... |
US Patent US9499523 (2016)
BindingDB Entry DOI: 10.7270/Q2S46QWR |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit delta isoform
(Homo sapiens (Human)) | BDBM255533
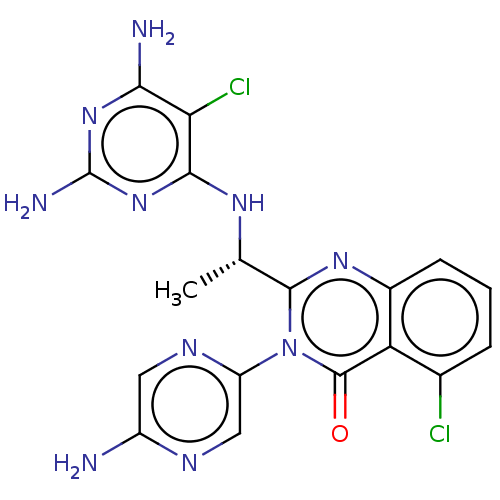
(US9499523, 7)Show SMILES C[C@H](Nc1nc(N)nc(N)c1Cl)c1nc2cccc(Cl)c2c(=O)n1-c1cnc(N)cn1 |r| Show InChI InChI=1S/C18H16Cl2N10O/c1-7(26-15-13(20)14(22)28-18(23)29-15)16-27-9-4-2-3-8(19)12(9)17(31)30(16)11-6-24-10(21)5-25-11/h2-7H,1H3,(H2,21,24)(H5,22,23,26,28,29)/t7-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | 24 | n/a | n/a | n/a | n/a | 7.4 | 25 |
GILEAD SCIENCES, INC.
US Patent
| Assay Description
The activities were measured using a time-resolved fluorescence resonance energy transfer (TR-FRET) assay. TR-FRET monitored the formation of 3,4,5-i... |
US Patent US9499523 (2016)
BindingDB Entry DOI: 10.7270/Q2S46QWR |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit delta isoform
(Homo sapiens (Human)) | BDBM255534
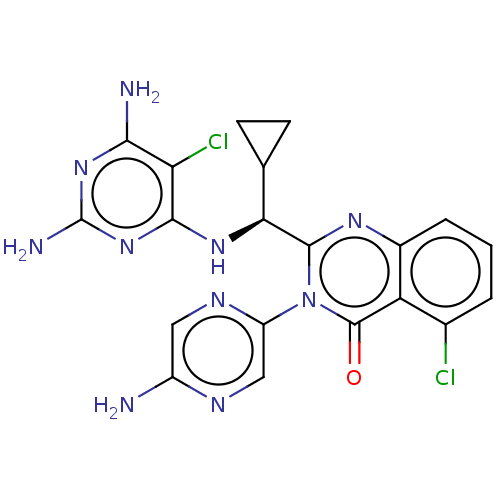
(US9499523, 8)Show SMILES Nc1cnc(cn1)-n1c(nc2cccc(Cl)c2c1=O)[C@@H](Nc1nc(N)nc(N)c1Cl)C1CC1 |r| Show InChI InChI=1S/C20H18Cl2N10O/c21-9-2-1-3-10-13(9)19(33)32(12-7-26-11(23)6-27-12)18(28-10)15(8-4-5-8)29-17-14(22)16(24)30-20(25)31-17/h1-3,6-8,15H,4-5H2,(H2,23,26)(H5,24,25,29,30,31)/t15-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | 24 | n/a | n/a | n/a | n/a | 7.4 | 25 |
GILEAD SCIENCES, INC.
US Patent
| Assay Description
The activities were measured using a time-resolved fluorescence resonance energy transfer (TR-FRET) assay. TR-FRET monitored the formation of 3,4,5-i... |
US Patent US9499523 (2016)
BindingDB Entry DOI: 10.7270/Q2S46QWR |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit delta isoform
(Homo sapiens (Human)) | BDBM255527

(US9499523, 1)Show SMILES C[C@H](Nc1nc(N)nc(N)c1Cl)c1nc2c(F)ccc(Cl)c2c(=O)n1-c1cnc(N)cn1 |r| Show InChI InChI=1S/C18H15Cl2FN10O/c1-6(27-15-12(20)14(23)29-18(24)30-15)16-28-13-8(21)3-2-7(19)11(13)17(32)31(16)10-5-25-9(22)4-26-10/h2-6H,1H3,(H2,22,25)(H5,23,24,27,29,30)/t6-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | 26 | n/a | n/a | n/a | n/a | 7.4 | 25 |
GILEAD SCIENCES, INC.
US Patent
| Assay Description
The activities were measured using a time-resolved fluorescence resonance energy transfer (TR-FRET) assay. TR-FRET monitored the formation of 3,4,5-i... |
US Patent US9499523 (2016)
BindingDB Entry DOI: 10.7270/Q2S46QWR |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit delta isoform
(Homo sapiens (Human)) | BDBM255546
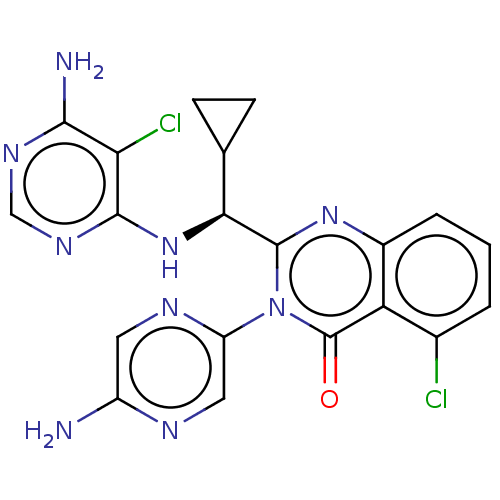
(US9499523, 20)Show SMILES Nc1cnc(cn1)-n1c(nc2cccc(Cl)c2c1=O)[C@@H](Nc1ncnc(N)c1Cl)C1CC1 |r| Show InChI InChI=1S/C20H17Cl2N9O/c21-10-2-1-3-11-14(10)20(32)31(13-7-25-12(23)6-26-13)19(29-11)16(9-4-5-9)30-18-15(22)17(24)27-8-28-18/h1-3,6-9,16H,4-5H2,(H2,23,25)(H3,24,27,28,30)/t16-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | 26 | n/a | n/a | n/a | n/a | 7.4 | 25 |
GILEAD SCIENCES, INC.
US Patent
| Assay Description
The activities were measured using a time-resolved fluorescence resonance energy transfer (TR-FRET) assay. TR-FRET monitored the formation of 3,4,5-i... |
US Patent US9499523 (2016)
BindingDB Entry DOI: 10.7270/Q2S46QWR |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit delta isoform
(Homo sapiens (Human)) | BDBM255547
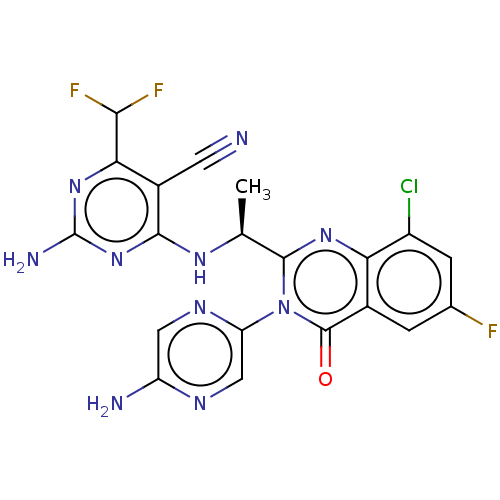
(US9499523, 21)Show SMILES C[C@H](Nc1nc(N)nc(C(F)F)c1C#N)c1nc2c(Cl)cc(F)cc2c(=O)n1-c1cnc(N)cn1 |r| Show InChI InChI=1S/C20H14ClF3N10O/c1-7(30-17-10(4-25)15(16(23)24)32-20(27)33-17)18-31-14-9(2-8(22)3-11(14)21)19(35)34(18)13-6-28-12(26)5-29-13/h2-3,5-7,16H,1H3,(H2,26,28)(H3,27,30,32,33)/t7-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | 30 | n/a | n/a | n/a | n/a | 7.4 | 25 |
GILEAD SCIENCES, INC.
US Patent
| Assay Description
The activities were measured using a time-resolved fluorescence resonance energy transfer (TR-FRET) assay. TR-FRET monitored the formation of 3,4,5-i... |
US Patent US9499523 (2016)
BindingDB Entry DOI: 10.7270/Q2S46QWR |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit delta isoform
(Homo sapiens (Human)) | BDBM255539
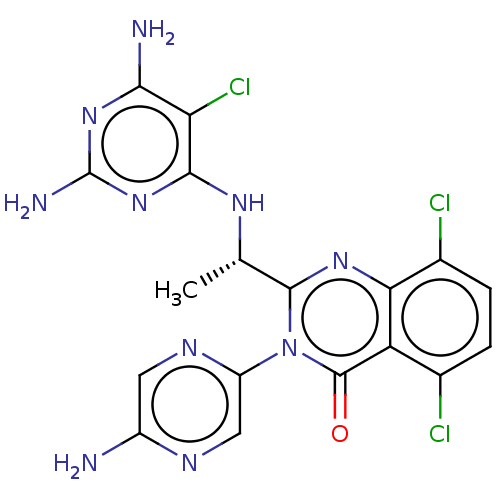
(US9499523, 13)Show SMILES C[C@H](Nc1nc(N)nc(N)c1Cl)c1nc2c(Cl)ccc(Cl)c2c(=O)n1-c1cnc(N)cn1 |r| Show InChI InChI=1S/C18H15Cl3N10O/c1-6(27-15-12(21)14(23)29-18(24)30-15)16-28-13-8(20)3-2-7(19)11(13)17(32)31(16)10-5-25-9(22)4-26-10/h2-6H,1H3,(H2,22,25)(H5,23,24,27,29,30)/t6-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | 31 | n/a | n/a | n/a | n/a | 7.4 | 25 |
GILEAD SCIENCES, INC.
US Patent
| Assay Description
The activities were measured using a time-resolved fluorescence resonance energy transfer (TR-FRET) assay. TR-FRET monitored the formation of 3,4,5-i... |
US Patent US9499523 (2016)
BindingDB Entry DOI: 10.7270/Q2S46QWR |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit delta isoform
(Homo sapiens (Human)) | BDBM255543

(US9499523, 17)Show SMILES C[C@H](Nc1nc(N)nc(N)c1C#N)c1nc2c(Cl)cc(F)cc2c(=O)n1-c1cnc(N)cn1 |r| Show InChI InChI=1S/C19H15ClFN11O/c1-7(28-16-10(4-22)15(24)30-19(25)31-16)17-29-14-9(2-8(21)3-11(14)20)18(33)32(17)13-6-26-12(23)5-27-13/h2-3,5-7H,1H3,(H2,23,26)(H5,24,25,28,30,31)/t7-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | 32 | n/a | n/a | n/a | n/a | 7.4 | 25 |
GILEAD SCIENCES, INC.
US Patent
| Assay Description
The activities were measured using a time-resolved fluorescence resonance energy transfer (TR-FRET) assay. TR-FRET monitored the formation of 3,4,5-i... |
US Patent US9499523 (2016)
BindingDB Entry DOI: 10.7270/Q2S46QWR |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit beta isoform
(Homo sapiens (Human)) | BDBM255533

(US9499523, 7)Show SMILES C[C@H](Nc1nc(N)nc(N)c1Cl)c1nc2cccc(Cl)c2c(=O)n1-c1cnc(N)cn1 |r| Show InChI InChI=1S/C18H16Cl2N10O/c1-7(26-15-13(20)14(22)28-18(23)29-15)16-27-9-4-2-3-8(19)12(9)17(31)30(16)11-6-24-10(21)5-25-11/h2-7H,1H3,(H2,21,24)(H5,22,23,26,28,29)/t7-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | 47 | n/a | n/a | n/a | n/a | 7.4 | 25 |
GILEAD SCIENCES, INC.
US Patent
| Assay Description
The activities were measured using a time-resolved fluorescence resonance energy transfer (TR-FRET) assay. TR-FRET monitored the formation of 3,4,5-i... |
US Patent US9499523 (2016)
BindingDB Entry DOI: 10.7270/Q2S46QWR |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit delta isoform
(Homo sapiens (Human)) | BDBM255541

(US9499523, 15)Show SMILES C[C@H](Nc1nc(N)nc(N)c1C#N)c1nc2c(F)cc(F)cc2c(=O)n1-c1cnc(N)cn1 |r| Show InChI InChI=1S/C19H15F2N11O/c1-7(28-16-10(4-22)15(24)30-19(25)31-16)17-29-14-9(2-8(20)3-11(14)21)18(33)32(17)13-6-26-12(23)5-27-13/h2-3,5-7H,1H3,(H2,23,26)(H5,24,25,28,30,31)/t7-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | 53 | n/a | n/a | n/a | n/a | 7.4 | 25 |
GILEAD SCIENCES, INC.
US Patent
| Assay Description
The activities were measured using a time-resolved fluorescence resonance energy transfer (TR-FRET) assay. TR-FRET monitored the formation of 3,4,5-i... |
US Patent US9499523 (2016)
BindingDB Entry DOI: 10.7270/Q2S46QWR |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit beta isoform
(Homo sapiens (Human)) | BDBM255527

(US9499523, 1)Show SMILES C[C@H](Nc1nc(N)nc(N)c1Cl)c1nc2c(F)ccc(Cl)c2c(=O)n1-c1cnc(N)cn1 |r| Show InChI InChI=1S/C18H15Cl2FN10O/c1-6(27-15-12(20)14(23)29-18(24)30-15)16-28-13-8(21)3-2-7(19)11(13)17(32)31(16)10-5-25-9(22)4-26-10/h2-6H,1H3,(H2,22,25)(H5,23,24,27,29,30)/t6-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | 54 | n/a | n/a | n/a | n/a | 7.4 | 25 |
GILEAD SCIENCES, INC.
US Patent
| Assay Description
The activities were measured using a time-resolved fluorescence resonance energy transfer (TR-FRET) assay. TR-FRET monitored the formation of 3,4,5-i... |
US Patent US9499523 (2016)
BindingDB Entry DOI: 10.7270/Q2S46QWR |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit delta isoform
(Homo sapiens (Human)) | BDBM255545

(US9499523, 19)Show SMILES Nc1cnc(cn1)-n1c(nc2c(Cl)ccc(Cl)c2c1=O)[C@@H](Nc1ncnc(N)c1Cl)C1CC1 |r| Show InChI InChI=1S/C20H16Cl3N9O/c21-9-3-4-10(22)16-13(9)20(33)32(12-6-26-11(24)5-27-12)19(31-16)15(8-1-2-8)30-18-14(23)17(25)28-7-29-18/h3-8,15H,1-2H2,(H2,24,26)(H3,25,28,29,30)/t15-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | 54 | n/a | n/a | n/a | n/a | 7.4 | 25 |
GILEAD SCIENCES, INC.
US Patent
| Assay Description
The activities were measured using a time-resolved fluorescence resonance energy transfer (TR-FRET) assay. TR-FRET monitored the formation of 3,4,5-i... |
US Patent US9499523 (2016)
BindingDB Entry DOI: 10.7270/Q2S46QWR |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit beta isoform
(Homo sapiens (Human)) | BDBM255529

(US9499523, 3)Show SMILES C[C@H](Nc1ncnc(N)c1C#N)c1nc2c(F)ccc(Cl)c2c(=O)n1-c1cnc(N)cn1 |r| Show InChI InChI=1S/C19H14ClFN10O/c1-8(29-17-9(4-22)16(24)27-7-28-17)18-30-15-11(21)3-2-10(20)14(15)19(32)31(18)13-6-25-12(23)5-26-13/h2-3,5-8H,1H3,(H2,23,25)(H3,24,27,28,29)/t8-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | 74 | n/a | n/a | n/a | n/a | 7.4 | 25 |
GILEAD SCIENCES, INC.
US Patent
| Assay Description
The activities were measured using a time-resolved fluorescence resonance energy transfer (TR-FRET) assay. TR-FRET monitored the formation of 3,4,5-i... |
US Patent US9499523 (2016)
BindingDB Entry DOI: 10.7270/Q2S46QWR |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit delta isoform
(Homo sapiens (Human)) | BDBM255538
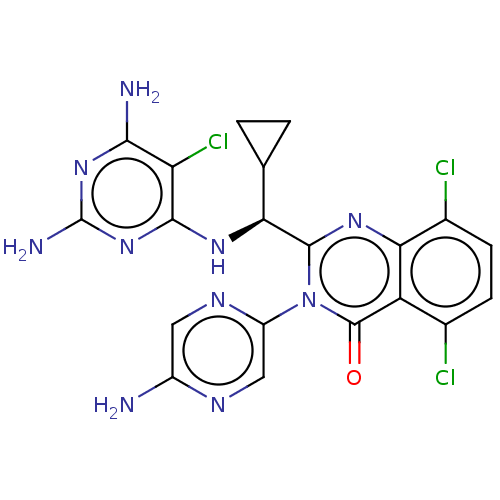
(US9499523, 12)Show SMILES Nc1cnc(cn1)-n1c(nc2c(Cl)ccc(Cl)c2c1=O)[C@@H](Nc1nc(N)nc(N)c1Cl)C1CC1 |r| Show InChI InChI=1S/C20H17Cl3N10O/c21-8-3-4-9(22)15-12(8)19(34)33(11-6-27-10(24)5-28-11)18(30-15)14(7-1-2-7)29-17-13(23)16(25)31-20(26)32-17/h3-7,14H,1-2H2,(H2,24,27)(H5,25,26,29,31,32)/t14-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | 97 | n/a | n/a | n/a | n/a | 7.4 | 25 |
GILEAD SCIENCES, INC.
US Patent
| Assay Description
The activities were measured using a time-resolved fluorescence resonance energy transfer (TR-FRET) assay. TR-FRET monitored the formation of 3,4,5-i... |
US Patent US9499523 (2016)
BindingDB Entry DOI: 10.7270/Q2S46QWR |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit beta isoform
(Homo sapiens (Human)) | BDBM255539

(US9499523, 13)Show SMILES C[C@H](Nc1nc(N)nc(N)c1Cl)c1nc2c(Cl)ccc(Cl)c2c(=O)n1-c1cnc(N)cn1 |r| Show InChI InChI=1S/C18H15Cl3N10O/c1-6(27-15-12(21)14(23)29-18(24)30-15)16-28-13-8(20)3-2-7(19)11(13)17(32)31(16)10-5-25-9(22)4-26-10/h2-6H,1H3,(H2,22,25)(H5,23,24,27,29,30)/t6-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | 170 | n/a | n/a | n/a | n/a | 7.4 | 25 |
GILEAD SCIENCES, INC.
US Patent
| Assay Description
The activities were measured using a time-resolved fluorescence resonance energy transfer (TR-FRET) assay. TR-FRET monitored the formation of 3,4,5-i... |
US Patent US9499523 (2016)
BindingDB Entry DOI: 10.7270/Q2S46QWR |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit beta isoform
(Homo sapiens (Human)) | BDBM255534

(US9499523, 8)Show SMILES Nc1cnc(cn1)-n1c(nc2cccc(Cl)c2c1=O)[C@@H](Nc1nc(N)nc(N)c1Cl)C1CC1 |r| Show InChI InChI=1S/C20H18Cl2N10O/c21-9-2-1-3-10-13(9)19(33)32(12-7-26-11(23)6-27-12)18(28-10)15(8-4-5-8)29-17-14(22)16(24)30-20(25)31-17/h1-3,6-8,15H,4-5H2,(H2,23,26)(H5,24,25,29,30,31)/t15-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | 190 | n/a | n/a | n/a | n/a | 7.4 | 25 |
GILEAD SCIENCES, INC.
US Patent
| Assay Description
The activities were measured using a time-resolved fluorescence resonance energy transfer (TR-FRET) assay. TR-FRET monitored the formation of 3,4,5-i... |
US Patent US9499523 (2016)
BindingDB Entry DOI: 10.7270/Q2S46QWR |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit beta isoform
(Homo sapiens (Human)) | BDBM255540
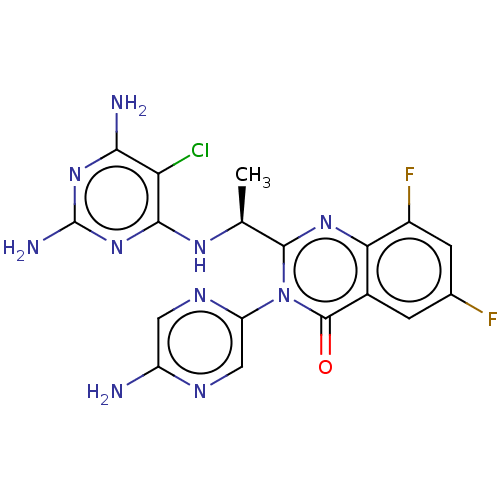
(US9499523, 14)Show SMILES C[C@H](Nc1nc(N)nc(N)c1Cl)c1nc2c(F)cc(F)cc2c(=O)n1-c1cnc(N)cn1 |r| Show InChI InChI=1S/C18H15ClF2N10O/c1-6(27-15-12(19)14(23)29-18(24)30-15)16-28-13-8(2-7(20)3-9(13)21)17(32)31(16)11-5-25-10(22)4-26-11/h2-6H,1H3,(H2,22,25)(H5,23,24,27,29,30)/t6-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | 200 | n/a | n/a | n/a | n/a | 7.4 | 25 |
GILEAD SCIENCES, INC.
US Patent
| Assay Description
The activities were measured using a time-resolved fluorescence resonance energy transfer (TR-FRET) assay. TR-FRET monitored the formation of 3,4,5-i... |
US Patent US9499523 (2016)
BindingDB Entry DOI: 10.7270/Q2S46QWR |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit gamma isoform
(Homo sapiens (Human)) | BDBM255532

(US9499523, 6)Show SMILES C[C@H](Nc1nc(N)nc(N)c1C#N)c1nc2cccc(Cl)c2c(=O)n1-c1cnc(N)cn1 |r| Show InChI InChI=1S/C19H16ClN11O/c1-8(27-16-9(5-21)15(23)29-19(24)30-16)17-28-11-4-2-3-10(20)14(11)18(32)31(17)13-7-25-12(22)6-26-13/h2-4,6-8H,1H3,(H2,22,25)(H5,23,24,27,29,30)/t8-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | 280 | n/a | n/a | n/a | n/a | 7.4 | 25 |
GILEAD SCIENCES, INC.
US Patent
| Assay Description
The activities were measured using a time-resolved fluorescence resonance energy transfer (TR-FRET) assay. TR-FRET monitored the formation of 3,4,5-i... |
US Patent US9499523 (2016)
BindingDB Entry DOI: 10.7270/Q2S46QWR |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit delta isoform
(Homo sapiens (Human)) | BDBM255540

(US9499523, 14)Show SMILES C[C@H](Nc1nc(N)nc(N)c1Cl)c1nc2c(F)cc(F)cc2c(=O)n1-c1cnc(N)cn1 |r| Show InChI InChI=1S/C18H15ClF2N10O/c1-6(27-15-12(19)14(23)29-18(24)30-15)16-28-13-8(2-7(20)3-9(13)21)17(32)31(16)11-5-25-10(22)4-26-11/h2-6H,1H3,(H2,22,25)(H5,23,24,27,29,30)/t6-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | 340 | n/a | n/a | n/a | n/a | 7.4 | 25 |
GILEAD SCIENCES, INC.
US Patent
| Assay Description
The activities were measured using a time-resolved fluorescence resonance energy transfer (TR-FRET) assay. TR-FRET monitored the formation of 3,4,5-i... |
US Patent US9499523 (2016)
BindingDB Entry DOI: 10.7270/Q2S46QWR |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit beta isoform
(Homo sapiens (Human)) | BDBM255545

(US9499523, 19)Show SMILES Nc1cnc(cn1)-n1c(nc2c(Cl)ccc(Cl)c2c1=O)[C@@H](Nc1ncnc(N)c1Cl)C1CC1 |r| Show InChI InChI=1S/C20H16Cl3N9O/c21-9-3-4-10(22)16-13(9)20(33)32(12-6-26-11(24)5-27-12)19(31-16)15(8-1-2-8)30-18-14(23)17(25)28-7-29-18/h3-8,15H,1-2H2,(H2,24,26)(H3,25,28,29,30)/t15-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | 360 | n/a | n/a | n/a | n/a | 7.4 | 25 |
GILEAD SCIENCES, INC.
US Patent
| Assay Description
The activities were measured using a time-resolved fluorescence resonance energy transfer (TR-FRET) assay. TR-FRET monitored the formation of 3,4,5-i... |
US Patent US9499523 (2016)
BindingDB Entry DOI: 10.7270/Q2S46QWR |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit delta isoform
(Homo sapiens (Human)) | BDBM255544
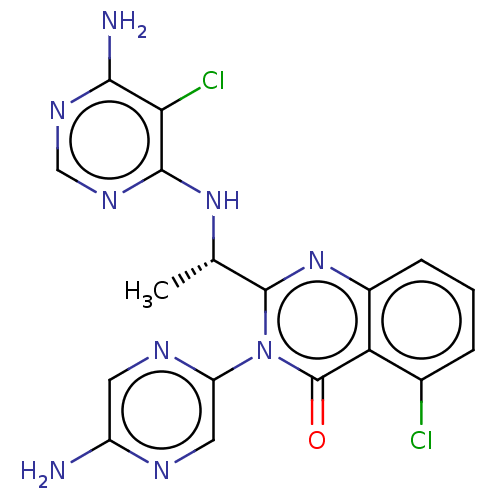
(US9499523, 18)Show SMILES C[C@H](Nc1ncnc(N)c1Cl)c1nc2cccc(Cl)c2c(=O)n1-c1cnc(N)cn1 |r| Show InChI InChI=1S/C18H15Cl2N9O/c1-8(27-16-14(20)15(22)25-7-26-16)17-28-10-4-2-3-9(19)13(10)18(30)29(17)12-6-23-11(21)5-24-12/h2-8H,1H3,(H2,21,23)(H3,22,25,26,27)/t8-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | 500 | n/a | n/a | n/a | n/a | 7.4 | 25 |
GILEAD SCIENCES, INC.
US Patent
| Assay Description
The activities were measured using a time-resolved fluorescence resonance energy transfer (TR-FRET) assay. TR-FRET monitored the formation of 3,4,5-i... |
US Patent US9499523 (2016)
BindingDB Entry DOI: 10.7270/Q2S46QWR |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit beta isoform
(Homo sapiens (Human)) | BDBM255542
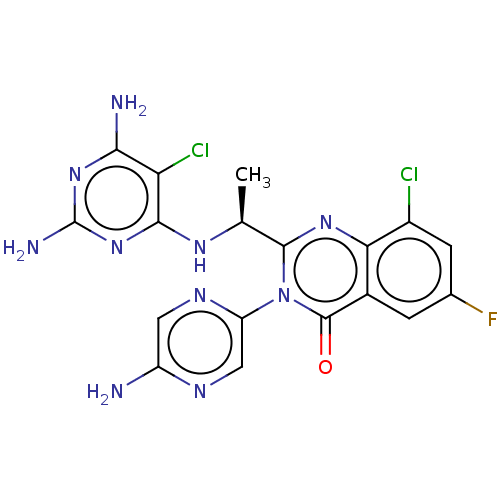
(US9499523, 16)Show SMILES C[C@H](Nc1nc(N)nc(N)c1Cl)c1nc2c(Cl)cc(F)cc2c(=O)n1-c1cnc(N)cn1 |r| Show InChI InChI=1S/C18H15Cl2FN10O/c1-6(27-15-12(20)14(23)29-18(24)30-15)16-28-13-8(2-7(21)3-9(13)19)17(32)31(16)11-5-25-10(22)4-26-11/h2-6H,1H3,(H2,22,25)(H5,23,24,27,29,30)/t6-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | 530 | n/a | n/a | n/a | n/a | 7.4 | 25 |
GILEAD SCIENCES, INC.
US Patent
| Assay Description
The activities were measured using a time-resolved fluorescence resonance energy transfer (TR-FRET) assay. TR-FRET monitored the formation of 3,4,5-i... |
US Patent US9499523 (2016)
BindingDB Entry DOI: 10.7270/Q2S46QWR |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit beta isoform
(Homo sapiens (Human)) | BDBM255544

(US9499523, 18)Show SMILES C[C@H](Nc1ncnc(N)c1Cl)c1nc2cccc(Cl)c2c(=O)n1-c1cnc(N)cn1 |r| Show InChI InChI=1S/C18H15Cl2N9O/c1-8(27-16-14(20)15(22)25-7-26-16)17-28-10-4-2-3-9(19)13(10)18(30)29(17)12-6-23-11(21)5-24-12/h2-8H,1H3,(H2,21,23)(H3,22,25,26,27)/t8-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | 570 | n/a | n/a | n/a | n/a | 7.4 | 25 |
GILEAD SCIENCES, INC.
US Patent
| Assay Description
The activities were measured using a time-resolved fluorescence resonance energy transfer (TR-FRET) assay. TR-FRET monitored the formation of 3,4,5-i... |
US Patent US9499523 (2016)
BindingDB Entry DOI: 10.7270/Q2S46QWR |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit gamma isoform
(Homo sapiens (Human)) | BDBM255528

(US9499523, 2)Show SMILES C[C@H](Nc1nc(N)nc(N)c1C#N)c1nc2c(F)ccc(Cl)c2c(=O)n1-c1cnc(N)cn1 |r| Show InChI InChI=1S/C19H15ClFN11O/c1-7(28-16-8(4-22)15(24)30-19(25)31-16)17-29-14-10(21)3-2-9(20)13(14)18(33)32(17)12-6-26-11(23)5-27-12/h2-3,5-7H,1H3,(H2,23,26)(H5,24,25,28,30,31)/t7-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | 630 | n/a | n/a | n/a | n/a | 7.4 | 25 |
GILEAD SCIENCES, INC.
US Patent
| Assay Description
The activities were measured using a time-resolved fluorescence resonance energy transfer (TR-FRET) assay. TR-FRET monitored the formation of 3,4,5-i... |
US Patent US9499523 (2016)
BindingDB Entry DOI: 10.7270/Q2S46QWR |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit beta isoform
(Homo sapiens (Human)) | BDBM255547

(US9499523, 21)Show SMILES C[C@H](Nc1nc(N)nc(C(F)F)c1C#N)c1nc2c(Cl)cc(F)cc2c(=O)n1-c1cnc(N)cn1 |r| Show InChI InChI=1S/C20H14ClF3N10O/c1-7(30-17-10(4-25)15(16(23)24)32-20(27)33-17)18-31-14-9(2-8(22)3-11(14)21)19(35)34(18)13-6-28-12(26)5-29-13/h2-3,5-7,16H,1H3,(H2,26,28)(H3,27,30,32,33)/t7-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | 720 | n/a | n/a | n/a | n/a | 7.4 | 25 |
GILEAD SCIENCES, INC.
US Patent
| Assay Description
The activities were measured using a time-resolved fluorescence resonance energy transfer (TR-FRET) assay. TR-FRET monitored the formation of 3,4,5-i... |
US Patent US9499523 (2016)
BindingDB Entry DOI: 10.7270/Q2S46QWR |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit delta isoform
(Homo sapiens (Human)) | BDBM255542

(US9499523, 16)Show SMILES C[C@H](Nc1nc(N)nc(N)c1Cl)c1nc2c(Cl)cc(F)cc2c(=O)n1-c1cnc(N)cn1 |r| Show InChI InChI=1S/C18H15Cl2FN10O/c1-6(27-15-12(20)14(23)29-18(24)30-15)16-28-13-8(2-7(21)3-9(13)19)17(32)31(16)11-5-25-10(22)4-26-11/h2-6H,1H3,(H2,22,25)(H5,23,24,27,29,30)/t6-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | 780 | n/a | n/a | n/a | n/a | 7.4 | 25 |
GILEAD SCIENCES, INC.
US Patent
| Assay Description
The activities were measured using a time-resolved fluorescence resonance energy transfer (TR-FRET) assay. TR-FRET monitored the formation of 3,4,5-i... |
US Patent US9499523 (2016)
BindingDB Entry DOI: 10.7270/Q2S46QWR |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit gamma isoform
(Homo sapiens (Human)) | BDBM255535

(US9499523, 9)Show SMILES Nc1cnc(cn1)-n1c(nc2cccc(Cl)c2c1=O)[C@@H](Nc1nc(N)nc(N)c1C#N)C1CC1 |r| Show InChI InChI=1S/C21H18ClN11O/c22-11-2-1-3-12-15(11)20(34)33(14-8-27-13(24)7-28-14)19(29-12)16(9-4-5-9)30-18-10(6-23)17(25)31-21(26)32-18/h1-3,7-9,16H,4-5H2,(H2,24,27)(H5,25,26,30,31,32)/t16-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | 900 | n/a | n/a | n/a | n/a | 7.4 | 25 |
GILEAD SCIENCES, INC.
US Patent
| Assay Description
The activities were measured using a time-resolved fluorescence resonance energy transfer (TR-FRET) assay. TR-FRET monitored the formation of 3,4,5-i... |
US Patent US9499523 (2016)
BindingDB Entry DOI: 10.7270/Q2S46QWR |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit beta isoform
(Homo sapiens (Human)) | BDBM255546

(US9499523, 20)Show SMILES Nc1cnc(cn1)-n1c(nc2cccc(Cl)c2c1=O)[C@@H](Nc1ncnc(N)c1Cl)C1CC1 |r| Show InChI InChI=1S/C20H17Cl2N9O/c21-10-2-1-3-11-14(10)20(32)31(13-7-25-12(23)6-26-13)19(29-11)16(9-4-5-9)30-18-15(22)17(24)27-8-28-18/h1-3,6-9,16H,4-5H2,(H2,23,25)(H3,24,27,28,30)/t16-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | 1.10E+3 | n/a | n/a | n/a | n/a | 7.4 | 25 |
GILEAD SCIENCES, INC.
US Patent
| Assay Description
The activities were measured using a time-resolved fluorescence resonance energy transfer (TR-FRET) assay. TR-FRET monitored the formation of 3,4,5-i... |
US Patent US9499523 (2016)
BindingDB Entry DOI: 10.7270/Q2S46QWR |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit beta isoform
(Homo sapiens (Human)) | BDBM255538

(US9499523, 12)Show SMILES Nc1cnc(cn1)-n1c(nc2c(Cl)ccc(Cl)c2c1=O)[C@@H](Nc1nc(N)nc(N)c1Cl)C1CC1 |r| Show InChI InChI=1S/C20H17Cl3N10O/c21-8-3-4-9(22)15-12(8)19(34)33(11-6-27-10(24)5-28-11)18(30-15)14(7-1-2-7)29-17-13(23)16(25)31-20(26)32-17/h3-7,14H,1-2H2,(H2,24,27)(H5,25,26,29,31,32)/t14-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | 1.20E+3 | n/a | n/a | n/a | n/a | 7.4 | 25 |
GILEAD SCIENCES, INC.
US Patent
| Assay Description
The activities were measured using a time-resolved fluorescence resonance energy transfer (TR-FRET) assay. TR-FRET monitored the formation of 3,4,5-i... |
US Patent US9499523 (2016)
BindingDB Entry DOI: 10.7270/Q2S46QWR |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit gamma isoform
(Homo sapiens (Human)) | BDBM255541

(US9499523, 15)Show SMILES C[C@H](Nc1nc(N)nc(N)c1C#N)c1nc2c(F)cc(F)cc2c(=O)n1-c1cnc(N)cn1 |r| Show InChI InChI=1S/C19H15F2N11O/c1-7(28-16-10(4-22)15(24)30-19(25)31-16)17-29-14-9(2-8(20)3-11(14)21)18(33)32(17)13-6-26-12(23)5-27-13/h2-3,5-7H,1H3,(H2,23,26)(H5,24,25,28,30,31)/t7-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | 1.50E+3 | n/a | n/a | n/a | n/a | 7.4 | 25 |
GILEAD SCIENCES, INC.
US Patent
| Assay Description
The activities were measured using a time-resolved fluorescence resonance energy transfer (TR-FRET) assay. TR-FRET monitored the formation of 3,4,5-i... |
US Patent US9499523 (2016)
BindingDB Entry DOI: 10.7270/Q2S46QWR |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit gamma isoform
(Homo sapiens (Human)) | BDBM255537

(US9499523, 11)Show SMILES C[C@H](Nc1nc(N)nc(N)c1C#N)c1nc2c(Cl)ccc(Cl)c2c(=O)n1-c1cnc(N)cn1 |r| Show InChI InChI=1S/C19H15Cl2N11O/c1-7(28-16-8(4-22)15(24)30-19(25)31-16)17-29-14-10(21)3-2-9(20)13(14)18(33)32(17)12-6-26-11(23)5-27-12/h2-3,5-7H,1H3,(H2,23,26)(H5,24,25,28,30,31)/t7-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | 1.50E+3 | n/a | n/a | n/a | n/a | 7.4 | 25 |
GILEAD SCIENCES, INC.
US Patent
| Assay Description
The activities were measured using a time-resolved fluorescence resonance energy transfer (TR-FRET) assay. TR-FRET monitored the formation of 3,4,5-i... |
US Patent US9499523 (2016)
BindingDB Entry DOI: 10.7270/Q2S46QWR |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit delta isoform
(Homo sapiens (Human)) | BDBM255531
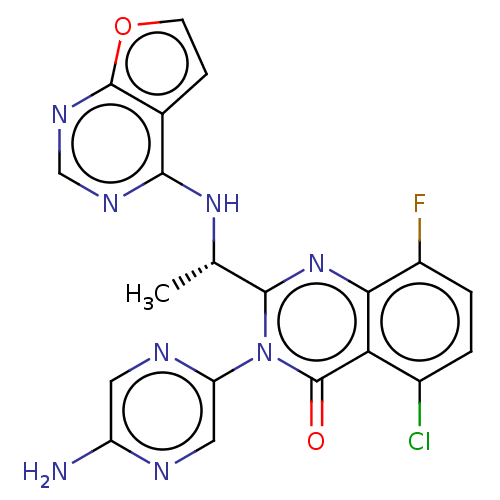
(US9499523, 5)Show SMILES C[C@H](Nc1ncnc2occc12)c1nc2c(F)ccc(Cl)c2c(=O)n1-c1cnc(N)cn1 |r| Show InChI InChI=1S/C20H14ClFN8O2/c1-9(28-17-10-4-5-32-19(10)27-8-26-17)18-29-16-12(22)3-2-11(21)15(16)20(31)30(18)14-7-24-13(23)6-25-14/h2-9H,1H3,(H2,23,24)(H,26,27,28)/t9-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | 2.30E+3 | n/a | n/a | n/a | n/a | 7.4 | 25 |
GILEAD SCIENCES, INC.
US Patent
| Assay Description
The activities were measured using a time-resolved fluorescence resonance energy transfer (TR-FRET) assay. TR-FRET monitored the formation of 3,4,5-i... |
US Patent US9499523 (2016)
BindingDB Entry DOI: 10.7270/Q2S46QWR |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit delta isoform
(Homo sapiens (Human)) | BDBM255530
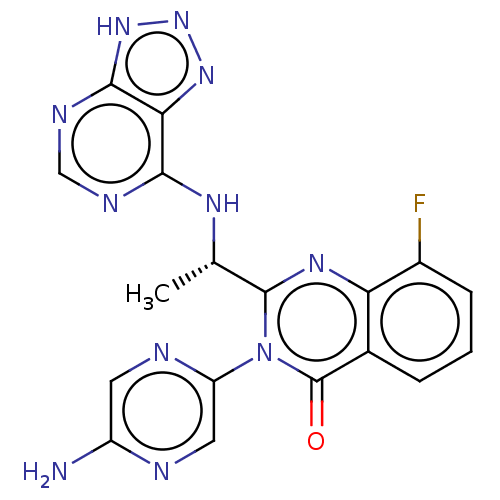
(US9499523, 4)Show SMILES C[C@H](Nc1ncnc2[nH]nnc12)c1nc2c(F)cccc2c(=O)n1-c1cnc(N)cn1 |r| | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | 2.70E+3 | n/a | n/a | n/a | n/a | 7.4 | 25 |
GILEAD SCIENCES, INC.
US Patent
| Assay Description
The activities were measured using a time-resolved fluorescence resonance energy transfer (TR-FRET) assay. TR-FRET monitored the formation of 3,4,5-i... |
US Patent US9499523 (2016)
BindingDB Entry DOI: 10.7270/Q2S46QWR |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit gamma isoform
(Homo sapiens (Human)) | BDBM255536

(US9499523, 10)Show SMILES Nc1cnc(cn1)-n1c(nc2c(Cl)ccc(Cl)c2c1=O)[C@@H](Nc1nc(N)nc(N)c1C#N)C1CC1 |r| Show InChI InChI=1S/C21H17Cl2N11O/c22-10-3-4-11(23)16-14(10)20(35)34(13-7-28-12(25)6-29-13)19(31-16)15(8-1-2-8)30-18-9(5-24)17(26)32-21(27)33-18/h3-4,6-8,15H,1-2H2,(H2,25,28)(H5,26,27,30,32,33)/t15-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | 3.10E+3 | n/a | n/a | n/a | n/a | 7.4 | 25 |
GILEAD SCIENCES, INC.
US Patent
| Assay Description
The activities were measured using a time-resolved fluorescence resonance energy transfer (TR-FRET) assay. TR-FRET monitored the formation of 3,4,5-i... |
US Patent US9499523 (2016)
BindingDB Entry DOI: 10.7270/Q2S46QWR |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit gamma isoform
(Homo sapiens (Human)) | BDBM255533

(US9499523, 7)Show SMILES C[C@H](Nc1nc(N)nc(N)c1Cl)c1nc2cccc(Cl)c2c(=O)n1-c1cnc(N)cn1 |r| Show InChI InChI=1S/C18H16Cl2N10O/c1-7(26-15-13(20)14(22)28-18(23)29-15)16-27-9-4-2-3-8(19)12(9)17(31)30(16)11-6-24-10(21)5-25-11/h2-7H,1H3,(H2,21,24)(H5,22,23,26,28,29)/t7-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | 4.00E+3 | n/a | n/a | n/a | n/a | 7.4 | 25 |
GILEAD SCIENCES, INC.
US Patent
| Assay Description
The activities were measured using a time-resolved fluorescence resonance energy transfer (TR-FRET) assay. TR-FRET monitored the formation of 3,4,5-i... |
US Patent US9499523 (2016)
BindingDB Entry DOI: 10.7270/Q2S46QWR |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit gamma isoform
(Homo sapiens (Human)) | BDBM255543

(US9499523, 17)Show SMILES C[C@H](Nc1nc(N)nc(N)c1C#N)c1nc2c(Cl)cc(F)cc2c(=O)n1-c1cnc(N)cn1 |r| Show InChI InChI=1S/C19H15ClFN11O/c1-7(28-16-10(4-22)15(24)30-19(25)31-16)17-29-14-9(2-8(21)3-11(14)20)18(33)32(17)13-6-26-12(23)5-27-13/h2-3,5-7H,1H3,(H2,23,26)(H5,24,25,28,30,31)/t7-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | 4.90E+3 | n/a | n/a | n/a | n/a | 7.4 | 25 |
GILEAD SCIENCES, INC.
US Patent
| Assay Description
The activities were measured using a time-resolved fluorescence resonance energy transfer (TR-FRET) assay. TR-FRET monitored the formation of 3,4,5-i... |
US Patent US9499523 (2016)
BindingDB Entry DOI: 10.7270/Q2S46QWR |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit beta isoform
(Homo sapiens (Human)) | BDBM255531

(US9499523, 5)Show SMILES C[C@H](Nc1ncnc2occc12)c1nc2c(F)ccc(Cl)c2c(=O)n1-c1cnc(N)cn1 |r| Show InChI InChI=1S/C20H14ClFN8O2/c1-9(28-17-10-4-5-32-19(10)27-8-26-17)18-29-16-12(22)3-2-11(21)15(16)20(31)30(18)14-7-24-13(23)6-25-14/h2-9H,1H3,(H2,23,24)(H,26,27,28)/t9-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | 5.80E+3 | n/a | n/a | n/a | n/a | 7.4 | 25 |
GILEAD SCIENCES, INC.
US Patent
| Assay Description
The activities were measured using a time-resolved fluorescence resonance energy transfer (TR-FRET) assay. TR-FRET monitored the formation of 3,4,5-i... |
US Patent US9499523 (2016)
BindingDB Entry DOI: 10.7270/Q2S46QWR |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit gamma isoform
(Homo sapiens (Human)) | BDBM255547

(US9499523, 21)Show SMILES C[C@H](Nc1nc(N)nc(C(F)F)c1C#N)c1nc2c(Cl)cc(F)cc2c(=O)n1-c1cnc(N)cn1 |r| Show InChI InChI=1S/C20H14ClF3N10O/c1-7(30-17-10(4-25)15(16(23)24)32-20(27)33-17)18-31-14-9(2-8(22)3-11(14)21)19(35)34(18)13-6-28-12(26)5-29-13/h2-3,5-7,16H,1H3,(H2,26,28)(H3,27,30,32,33)/t7-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | 7.4 | 25 |
GILEAD SCIENCES, INC.
US Patent
| Assay Description
The activities were measured using a time-resolved fluorescence resonance energy transfer (TR-FRET) assay. TR-FRET monitored the formation of 3,4,5-i... |
US Patent US9499523 (2016)
BindingDB Entry DOI: 10.7270/Q2S46QWR |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit gamma isoform
(Homo sapiens (Human)) | BDBM255542

(US9499523, 16)Show SMILES C[C@H](Nc1nc(N)nc(N)c1Cl)c1nc2c(Cl)cc(F)cc2c(=O)n1-c1cnc(N)cn1 |r| Show InChI InChI=1S/C18H15Cl2FN10O/c1-6(27-15-12(20)14(23)29-18(24)30-15)16-28-13-8(2-7(21)3-9(13)19)17(32)31(16)11-5-25-10(22)4-26-11/h2-6H,1H3,(H2,22,25)(H5,23,24,27,29,30)/t6-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | 7.4 | 25 |
GILEAD SCIENCES, INC.
US Patent
| Assay Description
The activities were measured using a time-resolved fluorescence resonance energy transfer (TR-FRET) assay. TR-FRET monitored the formation of 3,4,5-i... |
US Patent US9499523 (2016)
BindingDB Entry DOI: 10.7270/Q2S46QWR |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit gamma isoform
(Homo sapiens (Human)) | BDBM255544

(US9499523, 18)Show SMILES C[C@H](Nc1ncnc(N)c1Cl)c1nc2cccc(Cl)c2c(=O)n1-c1cnc(N)cn1 |r| Show InChI InChI=1S/C18H15Cl2N9O/c1-8(27-16-14(20)15(22)25-7-26-16)17-28-10-4-2-3-9(19)13(10)18(30)29(17)12-6-23-11(21)5-24-12/h2-8H,1H3,(H2,21,23)(H3,22,25,26,27)/t8-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | 7.4 | 25 |
GILEAD SCIENCES, INC.
US Patent
| Assay Description
The activities were measured using a time-resolved fluorescence resonance energy transfer (TR-FRET) assay. TR-FRET monitored the formation of 3,4,5-i... |
US Patent US9499523 (2016)
BindingDB Entry DOI: 10.7270/Q2S46QWR |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit gamma isoform
(Homo sapiens (Human)) | BDBM255534

(US9499523, 8)Show SMILES Nc1cnc(cn1)-n1c(nc2cccc(Cl)c2c1=O)[C@@H](Nc1nc(N)nc(N)c1Cl)C1CC1 |r| Show InChI InChI=1S/C20H18Cl2N10O/c21-9-2-1-3-10-13(9)19(33)32(12-7-26-11(23)6-27-12)18(28-10)15(8-4-5-8)29-17-14(22)16(24)30-20(25)31-17/h1-3,6-8,15H,4-5H2,(H2,23,26)(H5,24,25,29,30,31)/t15-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | 7.4 | 25 |
GILEAD SCIENCES, INC.
US Patent
| Assay Description
The activities were measured using a time-resolved fluorescence resonance energy transfer (TR-FRET) assay. TR-FRET monitored the formation of 3,4,5-i... |
US Patent US9499523 (2016)
BindingDB Entry DOI: 10.7270/Q2S46QWR |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit gamma isoform
(Homo sapiens (Human)) | BDBM255540

(US9499523, 14)Show SMILES C[C@H](Nc1nc(N)nc(N)c1Cl)c1nc2c(F)cc(F)cc2c(=O)n1-c1cnc(N)cn1 |r| Show InChI InChI=1S/C18H15ClF2N10O/c1-6(27-15-12(19)14(23)29-18(24)30-15)16-28-13-8(2-7(20)3-9(13)21)17(32)31(16)11-5-25-10(22)4-26-11/h2-6H,1H3,(H2,22,25)(H5,23,24,27,29,30)/t6-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | 7.4 | 25 |
GILEAD SCIENCES, INC.
US Patent
| Assay Description
The activities were measured using a time-resolved fluorescence resonance energy transfer (TR-FRET) assay. TR-FRET monitored the formation of 3,4,5-i... |
US Patent US9499523 (2016)
BindingDB Entry DOI: 10.7270/Q2S46QWR |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit gamma isoform
(Homo sapiens (Human)) | BDBM255538

(US9499523, 12)Show SMILES Nc1cnc(cn1)-n1c(nc2c(Cl)ccc(Cl)c2c1=O)[C@@H](Nc1nc(N)nc(N)c1Cl)C1CC1 |r| Show InChI InChI=1S/C20H17Cl3N10O/c21-8-3-4-9(22)15-12(8)19(34)33(11-6-27-10(24)5-28-11)18(30-15)14(7-1-2-7)29-17-13(23)16(25)31-20(26)32-17/h3-7,14H,1-2H2,(H2,24,27)(H5,25,26,29,31,32)/t14-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | 7.4 | 25 |
GILEAD SCIENCES, INC.
US Patent
| Assay Description
The activities were measured using a time-resolved fluorescence resonance energy transfer (TR-FRET) assay. TR-FRET monitored the formation of 3,4,5-i... |
US Patent US9499523 (2016)
BindingDB Entry DOI: 10.7270/Q2S46QWR |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit gamma isoform
(Homo sapiens (Human)) | BDBM255531

(US9499523, 5)Show SMILES C[C@H](Nc1ncnc2occc12)c1nc2c(F)ccc(Cl)c2c(=O)n1-c1cnc(N)cn1 |r| Show InChI InChI=1S/C20H14ClFN8O2/c1-9(28-17-10-4-5-32-19(10)27-8-26-17)18-29-16-12(22)3-2-11(21)15(16)20(31)30(18)14-7-24-13(23)6-25-14/h2-9H,1H3,(H2,23,24)(H,26,27,28)/t9-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | 7.4 | 25 |
GILEAD SCIENCES, INC.
US Patent
| Assay Description
The activities were measured using a time-resolved fluorescence resonance energy transfer (TR-FRET) assay. TR-FRET monitored the formation of 3,4,5-i... |
US Patent US9499523 (2016)
BindingDB Entry DOI: 10.7270/Q2S46QWR |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit gamma isoform
(Homo sapiens (Human)) | BDBM255527

(US9499523, 1)Show SMILES C[C@H](Nc1nc(N)nc(N)c1Cl)c1nc2c(F)ccc(Cl)c2c(=O)n1-c1cnc(N)cn1 |r| Show InChI InChI=1S/C18H15Cl2FN10O/c1-6(27-15-12(20)14(23)29-18(24)30-15)16-28-13-8(21)3-2-7(19)11(13)17(32)31(16)10-5-25-9(22)4-26-10/h2-6H,1H3,(H2,22,25)(H5,23,24,27,29,30)/t6-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | 7.4 | 25 |
GILEAD SCIENCES, INC.
US Patent
| Assay Description
The activities were measured using a time-resolved fluorescence resonance energy transfer (TR-FRET) assay. TR-FRET monitored the formation of 3,4,5-i... |
US Patent US9499523 (2016)
BindingDB Entry DOI: 10.7270/Q2S46QWR |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit gamma isoform
(Homo sapiens (Human)) | BDBM255539

(US9499523, 13)Show SMILES C[C@H](Nc1nc(N)nc(N)c1Cl)c1nc2c(Cl)ccc(Cl)c2c(=O)n1-c1cnc(N)cn1 |r| Show InChI InChI=1S/C18H15Cl3N10O/c1-6(27-15-12(21)14(23)29-18(24)30-15)16-28-13-8(20)3-2-7(19)11(13)17(32)31(16)10-5-25-9(22)4-26-10/h2-6H,1H3,(H2,22,25)(H5,23,24,27,29,30)/t6-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | 7.4 | 25 |
GILEAD SCIENCES, INC.
US Patent
| Assay Description
The activities were measured using a time-resolved fluorescence resonance energy transfer (TR-FRET) assay. TR-FRET monitored the formation of 3,4,5-i... |
US Patent US9499523 (2016)
BindingDB Entry DOI: 10.7270/Q2S46QWR |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit gamma isoform
(Homo sapiens (Human)) | BDBM255546

(US9499523, 20)Show SMILES Nc1cnc(cn1)-n1c(nc2cccc(Cl)c2c1=O)[C@@H](Nc1ncnc(N)c1Cl)C1CC1 |r| Show InChI InChI=1S/C20H17Cl2N9O/c21-10-2-1-3-11-14(10)20(32)31(13-7-25-12(23)6-26-13)19(29-11)16(9-4-5-9)30-18-15(22)17(24)27-8-28-18/h1-3,6-9,16H,4-5H2,(H2,23,25)(H3,24,27,28,30)/t16-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | 7.4 | 25 |
GILEAD SCIENCES, INC.
US Patent
| Assay Description
The activities were measured using a time-resolved fluorescence resonance energy transfer (TR-FRET) assay. TR-FRET monitored the formation of 3,4,5-i... |
US Patent US9499523 (2016)
BindingDB Entry DOI: 10.7270/Q2S46QWR |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit gamma isoform
(Homo sapiens (Human)) | BDBM255530

(US9499523, 4)Show SMILES C[C@H](Nc1ncnc2[nH]nnc12)c1nc2c(F)cccc2c(=O)n1-c1cnc(N)cn1 |r| | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | 7.4 | 25 |
GILEAD SCIENCES, INC.
US Patent
| Assay Description
The activities were measured using a time-resolved fluorescence resonance energy transfer (TR-FRET) assay. TR-FRET monitored the formation of 3,4,5-i... |
US Patent US9499523 (2016)
BindingDB Entry DOI: 10.7270/Q2S46QWR |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit gamma isoform
(Homo sapiens (Human)) | BDBM255545

(US9499523, 19)Show SMILES Nc1cnc(cn1)-n1c(nc2c(Cl)ccc(Cl)c2c1=O)[C@@H](Nc1ncnc(N)c1Cl)C1CC1 |r| Show InChI InChI=1S/C20H16Cl3N9O/c21-9-3-4-10(22)16-13(9)20(33)32(12-6-26-11(24)5-27-12)19(31-16)15(8-1-2-8)30-18-14(23)17(25)28-7-29-18/h3-8,15H,1-2H2,(H2,24,26)(H3,25,28,29,30)/t15-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | 7.4 | 25 |
GILEAD SCIENCES, INC.
US Patent
| Assay Description
The activities were measured using a time-resolved fluorescence resonance energy transfer (TR-FRET) assay. TR-FRET monitored the formation of 3,4,5-i... |
US Patent US9499523 (2016)
BindingDB Entry DOI: 10.7270/Q2S46QWR |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit gamma isoform
(Homo sapiens (Human)) | BDBM255529

(US9499523, 3)Show SMILES C[C@H](Nc1ncnc(N)c1C#N)c1nc2c(F)ccc(Cl)c2c(=O)n1-c1cnc(N)cn1 |r| Show InChI InChI=1S/C19H14ClFN10O/c1-8(29-17-9(4-22)16(24)27-7-28-17)18-30-15-11(21)3-2-10(20)14(15)19(32)31(18)13-6-25-12(23)5-26-13/h2-3,5-8H,1H3,(H2,23,25)(H3,24,27,28,29)/t8-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | 7.4 | 25 |
GILEAD SCIENCES, INC.
US Patent
| Assay Description
The activities were measured using a time-resolved fluorescence resonance energy transfer (TR-FRET) assay. TR-FRET monitored the formation of 3,4,5-i... |
US Patent US9499523 (2016)
BindingDB Entry DOI: 10.7270/Q2S46QWR |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit beta isoform
(Homo sapiens (Human)) | BDBM255530

(US9499523, 4)Show SMILES C[C@H](Nc1ncnc2[nH]nnc12)c1nc2c(F)cccc2c(=O)n1-c1cnc(N)cn1 |r| | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | 7.4 | 25 |
GILEAD SCIENCES, INC.
US Patent
| Assay Description
The activities were measured using a time-resolved fluorescence resonance energy transfer (TR-FRET) assay. TR-FRET monitored the formation of 3,4,5-i... |
US Patent US9499523 (2016)
BindingDB Entry DOI: 10.7270/Q2S46QWR |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data