

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Cytochrome P450 3A4 (Homo sapiens (Human)) | BDBM151585 (US11739089, Compound Ketoconazole | US8987315, Ket...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase DrugBank PC cid PC sid PDB UniChem Similars | DrugBank PDB US Patent | n/a | n/a | 20 | n/a | n/a | n/a | n/a | 7.4 | n/a |
NMMLSC US Patent | Assay Description Specific aspects of the incubation conditions for each assay (e.g., protein concentration, incubation time, etc.) are defined in Walsky & Obach, 2004... | US Patent US9688624 (2017) BindingDB Entry DOI: 10.7270/Q2416V6H | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Cytochrome P450 3A4 (Homo sapiens (Human)) | BDBM151585 (US11739089, Compound Ketoconazole | US8987315, Ket...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase DrugBank PC cid PC sid PDB UniChem Similars | DrugBank PDB US Patent | n/a | n/a | 20 | n/a | n/a | n/a | n/a | 7.4 | n/a |
NMMLSC US Patent | Assay Description Specific aspects of the incubation conditions for each assay (e.g., protein concentration, incubation time, etc.) are defined in Walsky & Obach, 2004... | US Patent US9688624 (2017) BindingDB Entry DOI: 10.7270/Q2416V6H | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Cytochrome P450 2D6 (Homo sapiens (Human)) | BDBM190405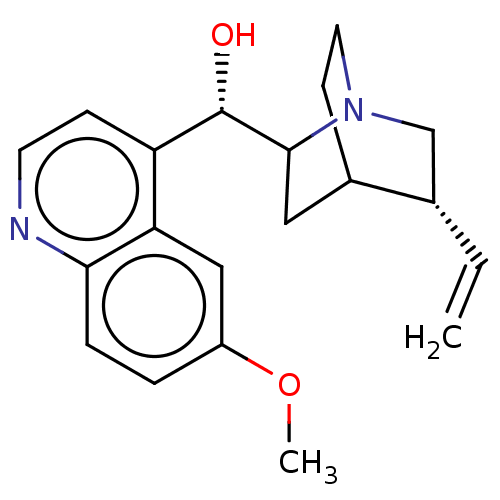 (US9180183, Quinidine) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | Purchase PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 40 | n/a | n/a | n/a | n/a | 7.4 | n/a |
NMMLSC US Patent | Assay Description Specific aspects of the incubation conditions for each assay (e.g., protein concentration, incubation time, etc.) are defined in Walsky & Obach, 2004... | US Patent US9688624 (2017) BindingDB Entry DOI: 10.7270/Q2416V6H | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 2C9 (Homo sapiens (Human)) | BDBM50090677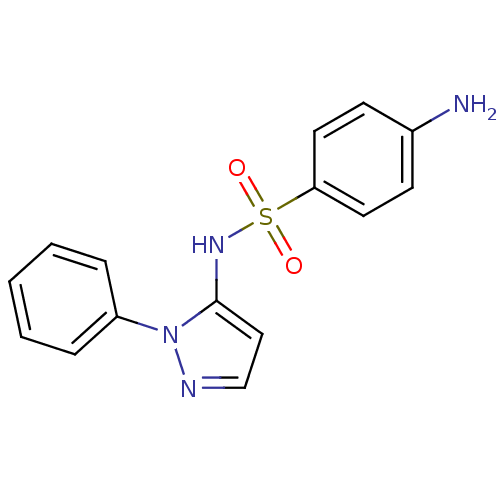 (4-Amino-N-(2-phenyl-2H-pyrazol-3-yl)-benzenesulfon...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE PC cid PC sid UniChem Patents Similars | DrugBank US Patent | n/a | n/a | 210 | n/a | n/a | n/a | n/a | 7.4 | n/a |
NMMLSC US Patent | Assay Description Specific aspects of the incubation conditions for each assay (e.g., protein concentration, incubation time, etc.) are defined in Walsky & Obach, 2004... | US Patent US9688624 (2017) BindingDB Entry DOI: 10.7270/Q2416V6H | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 1A2 (Homo sapiens (Human)) | BDBM50236897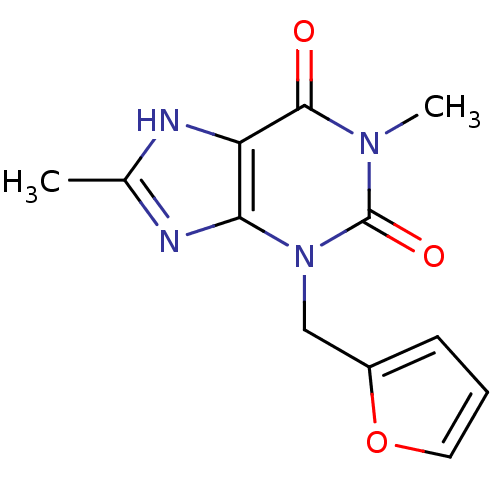 (3-(furan-2-ylmethyl)-1,8-dimethyl-1H-purine-2,6(3H...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 2.20E+3 | n/a | n/a | n/a | n/a | 7.4 | n/a |
NMMLSC US Patent | Assay Description Specific aspects of the incubation conditions for each assay (e.g., protein concentration, incubation time, etc.) are defined in Walsky & Obach, 2004... | US Patent US9688624 (2017) BindingDB Entry DOI: 10.7270/Q2416V6H | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 2C19 (Homo sapiens (Human)) | BDBM50267074 (CHEMBL446898 | N-3-benzyl-phenobarbital | Phenobar...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem | US Patent | n/a | n/a | 8.70E+3 | n/a | n/a | n/a | n/a | 7.4 | n/a |
NMMLSC US Patent | Assay Description Specific aspects of the incubation conditions for each assay (e.g., protein concentration, incubation time, etc.) are defined in Walsky & Obach, 2004... | US Patent US9688624 (2017) BindingDB Entry DOI: 10.7270/Q2416V6H | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 3A4 (Homo sapiens (Human)) | BDBM164620 (US9688624, Compound 2) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 2.60E+4 | n/a | n/a | n/a | n/a | 7.4 | n/a |
NMMLSC US Patent | Assay Description Specific aspects of the incubation conditions for each assay (e.g., protein concentration, incubation time, etc.) are defined in Walsky & Obach, 2004... | US Patent US9688624 (2017) BindingDB Entry DOI: 10.7270/Q2416V6H | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 3A4 (Homo sapiens (Human)) | BDBM164620 (US9688624, Compound 2) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | >5.00E+4 | n/a | n/a | n/a | n/a | 7.4 | n/a |
NMMLSC US Patent | Assay Description Specific aspects of the incubation conditions for each assay (e.g., protein concentration, incubation time, etc.) are defined in Walsky & Obach, 2004... | US Patent US9688624 (2017) BindingDB Entry DOI: 10.7270/Q2416V6H | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 1A2 (Homo sapiens (Human)) | BDBM164620 (US9688624, Compound 2) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | >5.00E+4 | n/a | n/a | n/a | n/a | 7.4 | n/a |
NMMLSC US Patent | Assay Description Specific aspects of the incubation conditions for each assay (e.g., protein concentration, incubation time, etc.) are defined in Walsky & Obach, 2004... | US Patent US9688624 (2017) BindingDB Entry DOI: 10.7270/Q2416V6H | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 2C9 (Homo sapiens (Human)) | BDBM164620 (US9688624, Compound 2) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | >5.00E+4 | n/a | n/a | n/a | n/a | 7.4 | n/a |
NMMLSC US Patent | Assay Description Specific aspects of the incubation conditions for each assay (e.g., protein concentration, incubation time, etc.) are defined in Walsky & Obach, 2004... | US Patent US9688624 (2017) BindingDB Entry DOI: 10.7270/Q2416V6H | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 2C19 (Homo sapiens (Human)) | BDBM164620 (US9688624, Compound 2) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | >5.00E+4 | n/a | n/a | n/a | n/a | 7.4 | n/a |
NMMLSC US Patent | Assay Description Specific aspects of the incubation conditions for each assay (e.g., protein concentration, incubation time, etc.) are defined in Walsky & Obach, 2004... | US Patent US9688624 (2017) BindingDB Entry DOI: 10.7270/Q2416V6H | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 2D6 (Homo sapiens (Human)) | BDBM164620 (US9688624, Compound 2) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | >5.00E+4 | n/a | n/a | n/a | n/a | 7.4 | n/a |
NMMLSC US Patent | Assay Description Specific aspects of the incubation conditions for each assay (e.g., protein concentration, incubation time, etc.) are defined in Walsky & Obach, 2004... | US Patent US9688624 (2017) BindingDB Entry DOI: 10.7270/Q2416V6H | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||