

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Aldo-keto reductase family 1 member B1 (315/316 > 99%)† (Homo sapiens (Human)) | BDBM16469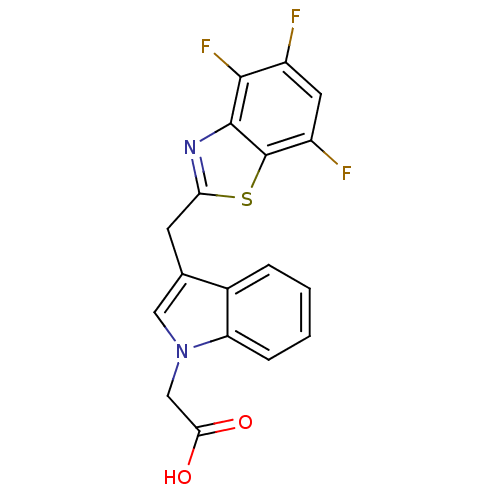 (2-{3-[(4,5,7-trifluoro-1,3-benzothiazol-2-yl)methy...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE MMDB PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | n/a | n/a | 5 | n/a | n/a | n/a | n/a | 6.6 | 37 |
The Institute for Diabetes Discovery | Assay Description The activity of the test enzyme was determined spectrophotometrically by monitoring the change in absorbance at 340 nm, which is due to the disappear... | J Med Chem 48: 3141-52 (2005) Article DOI: 10.1021/jm0492094 BindingDB Entry DOI: 10.7270/Q2J38QSN | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Aldo-keto reductase family 1 member B1 (269/315 = 85%)† (Rattus norvegicus) | BDBM16469 (2-{3-[(4,5,7-trifluoro-1,3-benzothiazol-2-yl)methy...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE MMDB PC cid PC sid PDB UniChem Patents Similars | MMDB PDB Article PubMed | n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
Slovak Academy of Sciences Curated by ChEMBL | Assay Description Inhibition of Wistar rat lens aldose reductase using D,L-glyceraldehyde as substrate incubated for 1 min measured for 4 mins by spectrophotometry | J Med Chem 58: 2649-57 (2015) Article DOI: 10.1021/jm5015814 BindingDB Entry DOI: 10.7270/Q2WS8VZJ | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Aldo-keto reductase family 1 member B1 (315/316 > 99%)† (Homo sapiens (Human)) | BDBM16469 (2-{3-[(4,5,7-trifluoro-1,3-benzothiazol-2-yl)methy...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE MMDB PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
Università di Modena e Reggio Emilia Curated by ChEMBL | Assay Description Inhibition of aldose reductase | Bioorg Med Chem 15: 7865-77 (2007) Article DOI: 10.1016/j.bmc.2007.08.019 BindingDB Entry DOI: 10.7270/Q2QR4WV6 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Aldo-keto reductase family 1 member B1 (315/316 > 99%)† (Homo sapiens (Human)) | BDBM16469 (2-{3-[(4,5,7-trifluoro-1,3-benzothiazol-2-yl)methy...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE MMDB PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | n/a | n/a | 2.70E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
The Institute for Diabetes Discovery, LLC Curated by ChEMBL | Assay Description Inhibition of human recombinant aldose reductase 1 after 10 mins by spectrophotometry analysis | Bioorg Med Chem Lett 19: 2006-8 (2009) Article DOI: 10.1016/j.bmcl.2009.02.037 BindingDB Entry DOI: 10.7270/Q2W66M3T | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||