

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Beta-lactamase (Staphylococcus aureus) | BDBM50079694 (CHEMBL294608 | Sodium; (2S,3R)-3-methyl-4,4,7-trio...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Southern Methodist University Curated by ChEMBL | Assay Description Inhibitory activity against serine Beta-lactamase derived from Staphylococcus aureus | Bioorg Med Chem Lett 9: 1997-2002 (1999) BindingDB Entry DOI: 10.7270/Q2TQ60RF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-lactamase (Staphylococcus aureus) | BDBM50079695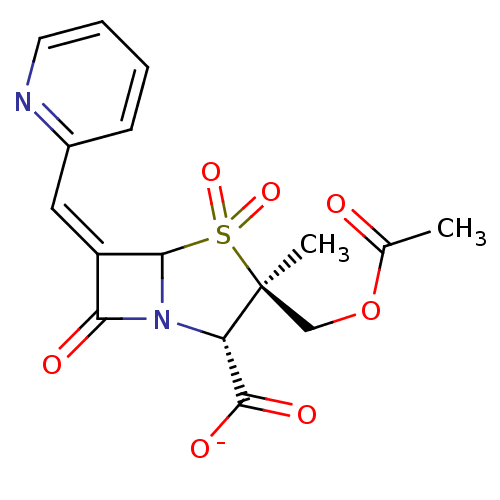 (CHEMBL294203 | sodium (2S,3R,5R,6Z)-3-[(acetyloxy)...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
Southern Methodist University Curated by ChEMBL | Assay Description Inhibitory activity against serine Beta-lactamase TEM derived from Staphylococcus aureus | Bioorg Med Chem Lett 9: 1997-2002 (1999) BindingDB Entry DOI: 10.7270/Q2TQ60RF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-lactamase (Staphylococcus aureus) | BDBM50079686 (CHEMBL292093 | disodium (2S,3R,5R,6Z)-3-methyl-6-(...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 15 | n/a | n/a | n/a | n/a | n/a | n/a |
Southern Methodist University Curated by ChEMBL | Assay Description Inhibitory activity against serine beta-lactamase, PC1 (class A) derived from Staphylococcus aureus | Bioorg Med Chem Lett 9: 1997-2002 (1999) BindingDB Entry DOI: 10.7270/Q2TQ60RF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-lactamase (Staphylococcus aureus) | BDBM50079689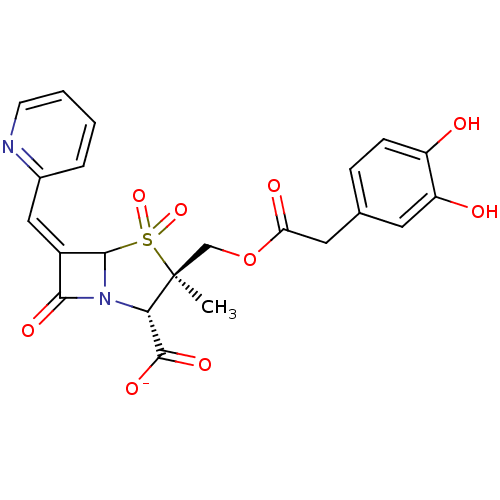 (CHEMBL303053 | Sodium; (2S,3R)-3-[2-(3,4-dihydroxy...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 26 | n/a | n/a | n/a | n/a | n/a | n/a |
Southern Methodist University Curated by ChEMBL | Assay Description Inhibitory activity against serine Beta-lactamase derived from Staphylococcus aureus | Bioorg Med Chem Lett 9: 1997-2002 (1999) BindingDB Entry DOI: 10.7270/Q2TQ60RF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-lactamase (Staphylococcus aureus) | BDBM50079694 (CHEMBL294608 | Sodium; (2S,3R)-3-methyl-4,4,7-trio...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 40 | n/a | n/a | n/a | n/a | n/a | n/a |
Southern Methodist University Curated by ChEMBL | Assay Description Inhibitory activity against serine Beta-lactamase derived from Staphylococcus aureus | Bioorg Med Chem Lett 9: 1997-2002 (1999) BindingDB Entry DOI: 10.7270/Q2TQ60RF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-lactamase (Staphylococcus aureus) | BDBM50079689 (CHEMBL303053 | Sodium; (2S,3R)-3-[2-(3,4-dihydroxy...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 60 | n/a | n/a | n/a | n/a | n/a | n/a |
Southern Methodist University Curated by ChEMBL | Assay Description Inhibitory activity against serine Beta-lactamase TEM derived from Staphylococcus aureus | Bioorg Med Chem Lett 9: 1997-2002 (1999) BindingDB Entry DOI: 10.7270/Q2TQ60RF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-lactamase (Staphylococcus aureus) | BDBM50079695 (CHEMBL294203 | sodium (2S,3R,5R,6Z)-3-[(acetyloxy)...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 62 | n/a | n/a | n/a | n/a | n/a | n/a |
Southern Methodist University Curated by ChEMBL | Assay Description Inhibitory activity against serine Beta-lactamase derived from Staphylococcus aureus | Bioorg Med Chem Lett 9: 1997-2002 (1999) BindingDB Entry DOI: 10.7270/Q2TQ60RF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-lactamase (Staphylococcus aureus) | BDBM50079684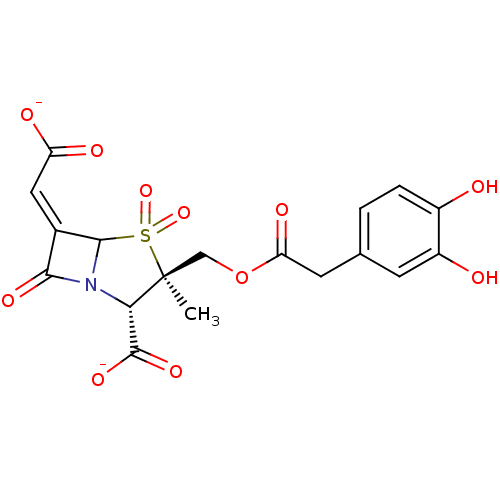 (CHEMBL305361 | disodium (2S,3R,5R,6Z)-3-({[(3,4-di...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 105 | n/a | n/a | n/a | n/a | n/a | n/a |
Southern Methodist University Curated by ChEMBL | Assay Description Inhibitory activity against serine Beta-lactamase TEM derived from Staphylococcus aureus | Bioorg Med Chem Lett 9: 1997-2002 (1999) BindingDB Entry DOI: 10.7270/Q2TQ60RF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-lactamase (Staphylococcus aureus) | BDBM50079685 (CHEMBL61762 | disodium (2S,3R,5R,6Z)-3-[(acetyloxy...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 180 | n/a | n/a | n/a | n/a | n/a | n/a |
Southern Methodist University Curated by ChEMBL | Assay Description Inhibitory activity against serine Beta-lactamase derived from Staphylococcus aureus | Bioorg Med Chem Lett 9: 1997-2002 (1999) BindingDB Entry DOI: 10.7270/Q2TQ60RF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-lactamase (Staphylococcus aureus) | BDBM50079682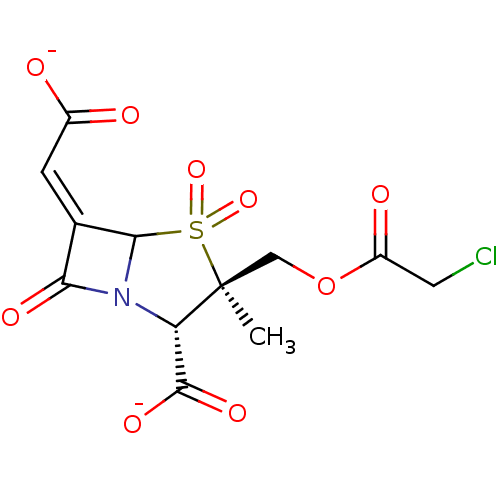 (CHEMBL64793 | disodium (2S,3R,5R,6Z)-3-{[(chloroac...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 196 | n/a | n/a | n/a | n/a | n/a | n/a |
Southern Methodist University Curated by ChEMBL | Assay Description Inhibitory activity against serine Beta-lactamase TEM derived from Staphylococcus aureus | Bioorg Med Chem Lett 9: 1997-2002 (1999) BindingDB Entry DOI: 10.7270/Q2TQ60RF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-lactamase (Staphylococcus aureus) | BDBM50079692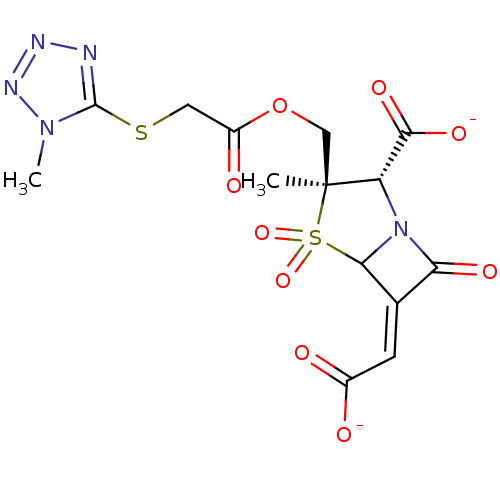 (CHEMBL292506 | Disodium; (2S,3R)-6-[1-carboxy-meth...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 233 | n/a | n/a | n/a | n/a | n/a | n/a |
Southern Methodist University Curated by ChEMBL | Assay Description Inhibitory activity against serine beta-lactamase, PC1 (class A) derived from Staphylococcus aureus | Bioorg Med Chem Lett 9: 1997-2002 (1999) BindingDB Entry DOI: 10.7270/Q2TQ60RF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-lactamase (Staphylococcus aureus) | BDBM50421413 (CL-307579 | TAZOBACTAM SODIUM | Tazocillin) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | Purchase MCE KEGG PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 297 | n/a | n/a | n/a | n/a | n/a | n/a |
Southern Methodist University Curated by ChEMBL | Assay Description Inhibitory activity against serine Beta-lactamase TEM derived from Staphylococcus aureus | Bioorg Med Chem Lett 9: 1997-2002 (1999) BindingDB Entry DOI: 10.7270/Q2TQ60RF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-lactamase (Staphylococcus aureus) | BDBM50079690 (CHEMBL304709 | sodium (2S,3R,5R,6Z)-3-[(acetyloxy)...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 310 | n/a | n/a | n/a | n/a | n/a | n/a |
Southern Methodist University Curated by ChEMBL | Assay Description Inhibitory activity against serine Beta-lactamase TEM derived from Staphylococcus aureus | Bioorg Med Chem Lett 9: 1997-2002 (1999) BindingDB Entry DOI: 10.7270/Q2TQ60RF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-lactamase (Staphylococcus aureus) | BDBM50079684 (CHEMBL305361 | disodium (2S,3R,5R,6Z)-3-({[(3,4-di...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 370 | n/a | n/a | n/a | n/a | n/a | n/a |
Southern Methodist University Curated by ChEMBL | Assay Description Inhibitory activity against serine Beta-lactamase derived from Staphylococcus aureus | Bioorg Med Chem Lett 9: 1997-2002 (1999) BindingDB Entry DOI: 10.7270/Q2TQ60RF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-lactamase (Staphylococcus aureus) | BDBM50079694 (CHEMBL294608 | Sodium; (2S,3R)-3-methyl-4,4,7-trio...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 390 | n/a | n/a | n/a | n/a | n/a | n/a |
Southern Methodist University Curated by ChEMBL | Assay Description Inhibitory activity against serine Beta-lactamase derived from Staphylococcus aureus | Bioorg Med Chem Lett 9: 1997-2002 (1999) BindingDB Entry DOI: 10.7270/Q2TQ60RF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-lactamase (Staphylococcus aureus) | BDBM50079686 (CHEMBL292093 | disodium (2S,3R,5R,6Z)-3-methyl-6-(...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 540 | n/a | n/a | n/a | n/a | n/a | n/a |
Southern Methodist University Curated by ChEMBL | Assay Description Inhibitory activity against serine beta-lactamase, PC1 (class A) derived from Staphylococcus aureus | Bioorg Med Chem Lett 9: 1997-2002 (1999) BindingDB Entry DOI: 10.7270/Q2TQ60RF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-lactamase (Staphylococcus aureus) | BDBM50079680 (CHEMBL61760 | disodium (2S,3R,5R,6Z)-3-[(formyloxy...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 592 | n/a | n/a | n/a | n/a | n/a | n/a |
Southern Methodist University Curated by ChEMBL | Assay Description Inhibitory activity against serine Beta-lactamase TEM derived from Staphylococcus aureus | Bioorg Med Chem Lett 9: 1997-2002 (1999) BindingDB Entry DOI: 10.7270/Q2TQ60RF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-lactamase (Staphylococcus aureus) | BDBM50079692 (CHEMBL292506 | Disodium; (2S,3R)-6-[1-carboxy-meth...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 640 | n/a | n/a | n/a | n/a | n/a | n/a |
Southern Methodist University Curated by ChEMBL | Assay Description Inhibitory activity against serine Beta-lactamase derived from Staphylococcus aureus | Bioorg Med Chem Lett 9: 1997-2002 (1999) BindingDB Entry DOI: 10.7270/Q2TQ60RF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-lactamase (Staphylococcus aureus) | BDBM50079695 (CHEMBL294203 | sodium (2S,3R,5R,6Z)-3-[(acetyloxy)...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 660 | n/a | n/a | n/a | n/a | n/a | n/a |
Southern Methodist University Curated by ChEMBL | Assay Description Inhibitory activity against serine Beta-lactamase TEM derived from Staphylococcus aureus | Bioorg Med Chem Lett 9: 1997-2002 (1999) BindingDB Entry DOI: 10.7270/Q2TQ60RF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-lactamase (Staphylococcus aureus) | BDBM50079689 (CHEMBL303053 | Sodium; (2S,3R)-3-[2-(3,4-dihydroxy...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 700 | n/a | n/a | n/a | n/a | n/a | n/a |
Southern Methodist University Curated by ChEMBL | Assay Description Inhibitory activity against serine Beta-lactamase TEM derived from Staphylococcus aureus | Bioorg Med Chem Lett 9: 1997-2002 (1999) BindingDB Entry DOI: 10.7270/Q2TQ60RF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-lactamase (Staphylococcus aureus) | BDBM50079685 (CHEMBL61762 | disodium (2S,3R,5R,6Z)-3-[(acetyloxy...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 708 | n/a | n/a | n/a | n/a | n/a | n/a |
Southern Methodist University Curated by ChEMBL | Assay Description Inhibitory activity against serine Beta-lactamase derived from Staphylococcus aureus | Bioorg Med Chem Lett 9: 1997-2002 (1999) BindingDB Entry DOI: 10.7270/Q2TQ60RF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-lactamase (Staphylococcus aureus) | BDBM50079691 (CHEMBL60491 | disodium (2S,5R,6Z)-3,3-dimethyl-6-(...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 1.80E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Southern Methodist University Curated by ChEMBL | Assay Description Inhibitory activity against serine Beta-lactamase derived from Staphylococcus aureus | Bioorg Med Chem Lett 9: 1997-2002 (1999) BindingDB Entry DOI: 10.7270/Q2TQ60RF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-lactamase (Staphylococcus aureus) | BDBM50079680 (CHEMBL61760 | disodium (2S,3R,5R,6Z)-3-[(formyloxy...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 1.84E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Southern Methodist University Curated by ChEMBL | Assay Description Inhibitory activity against serine Beta-lactamase TEM derived from Staphylococcus aureus | Bioorg Med Chem Lett 9: 1997-2002 (1999) BindingDB Entry DOI: 10.7270/Q2TQ60RF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-lactamase (Staphylococcus aureus) | BDBM50079690 (CHEMBL304709 | sodium (2S,3R,5R,6Z)-3-[(acetyloxy)...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 2.21E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Southern Methodist University Curated by ChEMBL | Assay Description Inhibitory activity against serine beta-lactamase, PC1 (class A) derived from Staphylococcus aureus | Bioorg Med Chem Lett 9: 1997-2002 (1999) BindingDB Entry DOI: 10.7270/Q2TQ60RF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-lactamase (Staphylococcus aureus) | BDBM50079693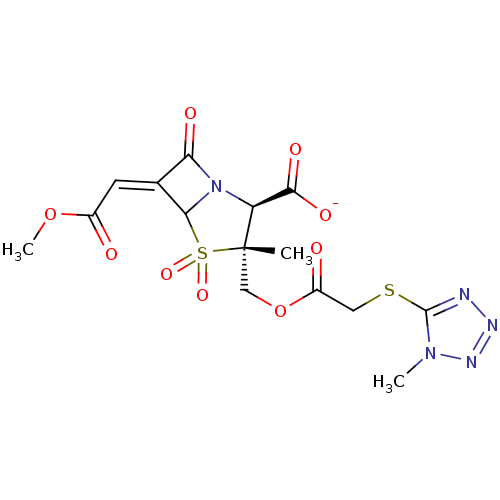 (CHEMBL59053 | Sodium; (2S,3R)-6-[1-methoxycarbonyl...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 2.37E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Southern Methodist University Curated by ChEMBL | Assay Description Inhibitory activity against serine Beta-lactamase TEM derived from Staphylococcus aureus | Bioorg Med Chem Lett 9: 1997-2002 (1999) BindingDB Entry DOI: 10.7270/Q2TQ60RF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-lactamase (Staphylococcus aureus) | BDBM50421413 (CL-307579 | TAZOBACTAM SODIUM | Tazocillin) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | Purchase MCE KEGG PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 2.57E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Southern Methodist University Curated by ChEMBL | Assay Description Inhibitory activity against serine Beta-lactamase TEM derived from Staphylococcus aureus | Bioorg Med Chem Lett 9: 1997-2002 (1999) BindingDB Entry DOI: 10.7270/Q2TQ60RF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-lactamase (Staphylococcus aureus) | BDBM50079679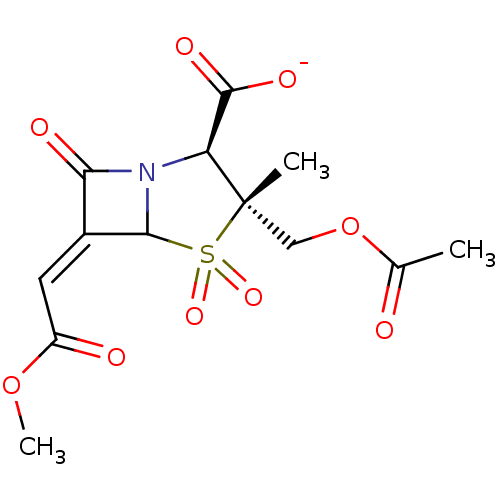 (CHEMBL64056 | sodium (2S,3R,5R,6Z)-3-[(acetyloxy)m...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 2.72E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Southern Methodist University Curated by ChEMBL | Assay Description Inhibitory activity against serine Beta-lactamase derived from Staphylococcus aureus | Bioorg Med Chem Lett 9: 1997-2002 (1999) BindingDB Entry DOI: 10.7270/Q2TQ60RF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-lactamase (Staphylococcus aureus) | BDBM50079691 (CHEMBL60491 | disodium (2S,5R,6Z)-3,3-dimethyl-6-(...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 4.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Southern Methodist University Curated by ChEMBL | Assay Description Inhibitory activity against serine beta-lactamase, PC1 (class A) derived from Staphylococcus aureus | Bioorg Med Chem Lett 9: 1997-2002 (1999) BindingDB Entry DOI: 10.7270/Q2TQ60RF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-lactamase (Staphylococcus aureus) | BDBM50079681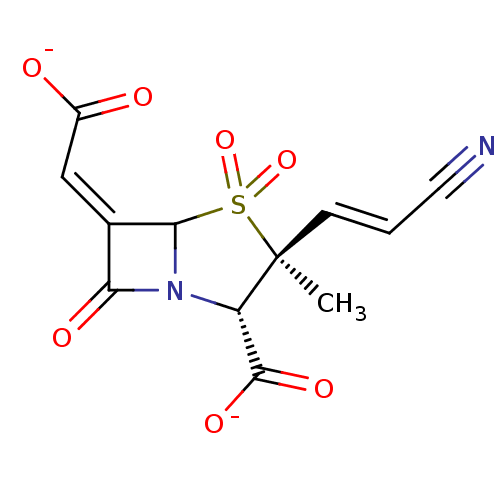 (CHEMBL60220 | disodium (2S,3S,5R,6Z)-3-[(E)-2-cyan...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 6.76E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Southern Methodist University Curated by ChEMBL | Assay Description Inhibitory activity against serine beta-lactamase, PC1 (class A) derived from Staphylococcus aureus | Bioorg Med Chem Lett 9: 1997-2002 (1999) BindingDB Entry DOI: 10.7270/Q2TQ60RF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-lactamase (Staphylococcus aureus) | BDBM50079682 (CHEMBL64793 | disodium (2S,3R,5R,6Z)-3-{[(chloroac...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 7.20E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Southern Methodist University Curated by ChEMBL | Assay Description Inhibitory activity against serine Beta-lactamase derived from Staphylococcus aureus | Bioorg Med Chem Lett 9: 1997-2002 (1999) BindingDB Entry DOI: 10.7270/Q2TQ60RF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-lactamase (Staphylococcus aureus) | BDBM50079690 (CHEMBL304709 | sodium (2S,3R,5R,6Z)-3-[(acetyloxy)...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 8.48E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Southern Methodist University Curated by ChEMBL | Assay Description Inhibitory activity against serine Beta-lactamase TEM derived from Staphylococcus aureus | Bioorg Med Chem Lett 9: 1997-2002 (1999) BindingDB Entry DOI: 10.7270/Q2TQ60RF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-lactamase (Staphylococcus aureus) | BDBM50079679 (CHEMBL64056 | sodium (2S,3R,5R,6Z)-3-[(acetyloxy)m...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 9.51E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Southern Methodist University Curated by ChEMBL | Assay Description Inhibitory activity against serine Beta-lactamase derived from Staphylococcus aureus | Bioorg Med Chem Lett 9: 1997-2002 (1999) BindingDB Entry DOI: 10.7270/Q2TQ60RF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-lactamase (Staphylococcus aureus) | BDBM50079693 (CHEMBL59053 | Sodium; (2S,3R)-6-[1-methoxycarbonyl...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 1.32E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Southern Methodist University Curated by ChEMBL | Assay Description Inhibitory activity against serine Beta-lactamase derived from Staphylococcus aureus | Bioorg Med Chem Lett 9: 1997-2002 (1999) BindingDB Entry DOI: 10.7270/Q2TQ60RF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-lactamase (Staphylococcus aureus) | BDBM50079683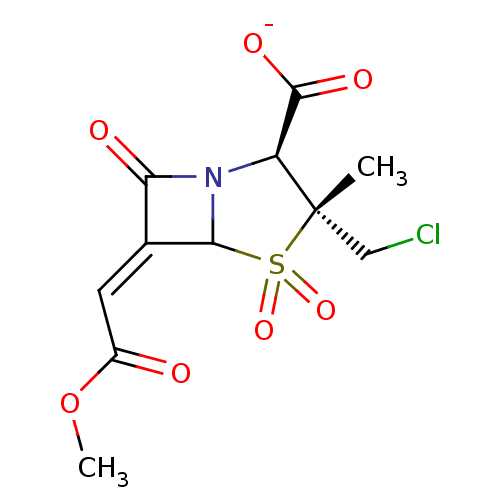 (CHEMBL292147 | Sodium; (2S,3R)-3-chloromethyl-6-[1...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 1.39E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Southern Methodist University Curated by ChEMBL | Assay Description Inhibitory activity against serine beta-lactamase, PC1 (class A) derived from Staphylococcus aureus | Bioorg Med Chem Lett 9: 1997-2002 (1999) BindingDB Entry DOI: 10.7270/Q2TQ60RF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-lactamase (Staphylococcus aureus) | BDBM50079681 (CHEMBL60220 | disodium (2S,3S,5R,6Z)-3-[(E)-2-cyan...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 2.17E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Southern Methodist University Curated by ChEMBL | Assay Description Inhibitory activity against serine Beta-lactamase TEM derived from Staphylococcus aureus | Bioorg Med Chem Lett 9: 1997-2002 (1999) BindingDB Entry DOI: 10.7270/Q2TQ60RF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-lactamase (Staphylococcus aureus) | BDBM50079683 (CHEMBL292147 | Sodium; (2S,3R)-3-chloromethyl-6-[1...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 4.45E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Southern Methodist University Curated by ChEMBL | Assay Description Inhibitory activity against serine Beta-lactamase TEM derived from Staphylococcus aureus | Bioorg Med Chem Lett 9: 1997-2002 (1999) BindingDB Entry DOI: 10.7270/Q2TQ60RF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-lactamase (Staphylococcus aureus) | BDBM50421413 (CL-307579 | TAZOBACTAM SODIUM | Tazocillin) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | Purchase MCE KEGG PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 5.19E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Southern Methodist University Curated by ChEMBL | Assay Description Inhibitory activity against serine Beta-lactamase derived from Staphylococcus aureus | Bioorg Med Chem Lett 9: 1997-2002 (1999) BindingDB Entry DOI: 10.7270/Q2TQ60RF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-lactamase (Staphylococcus aureus) | BDBM50079685 (CHEMBL61762 | disodium (2S,3R,5R,6Z)-3-[(acetyloxy...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 7.65E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Southern Methodist University Curated by ChEMBL | Assay Description Inhibitory activity against serine beta-lactamase, PC1 (class A) derived from Staphylococcus aureus | Bioorg Med Chem Lett 9: 1997-2002 (1999) BindingDB Entry DOI: 10.7270/Q2TQ60RF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-lactamase (Staphylococcus aureus) | BDBM50079691 (CHEMBL60491 | disodium (2S,5R,6Z)-3,3-dimethyl-6-(...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 1.08E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Southern Methodist University Curated by ChEMBL | Assay Description Inhibitory activity against serine beta-lactamase, PC1 (class A) derived from Staphylococcus aureus | Bioorg Med Chem Lett 9: 1997-2002 (1999) BindingDB Entry DOI: 10.7270/Q2TQ60RF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-lactamase (Staphylococcus aureus) | BDBM50079684 (CHEMBL305361 | disodium (2S,3R,5R,6Z)-3-({[(3,4-di...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 1.16E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Southern Methodist University Curated by ChEMBL | Assay Description Inhibitory activity against serine Beta-lactamase derived from Staphylococcus aureus | Bioorg Med Chem Lett 9: 1997-2002 (1999) BindingDB Entry DOI: 10.7270/Q2TQ60RF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-lactamase (Staphylococcus aureus) | BDBM50079688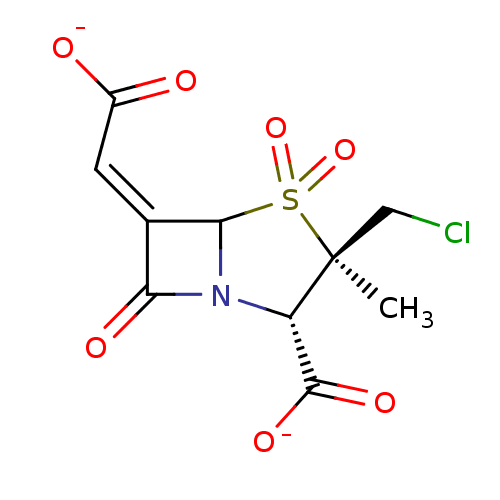 ((2S,3R,5R,6Z)-3-(chloromethyl)-3-methyl-6-(2-oxido...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 1.21E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Southern Methodist University Curated by ChEMBL | Assay Description Inhibitory activity against serine Beta-lactamase TEM derived from Staphylococcus aureus | Bioorg Med Chem Lett 9: 1997-2002 (1999) BindingDB Entry DOI: 10.7270/Q2TQ60RF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-lactamase (Staphylococcus aureus) | BDBM50079680 (CHEMBL61760 | disodium (2S,3R,5R,6Z)-3-[(formyloxy...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 1.73E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Southern Methodist University Curated by ChEMBL | Assay Description Inhibitory activity against serine Beta-lactamase TEM derived from Staphylococcus aureus | Bioorg Med Chem Lett 9: 1997-2002 (1999) BindingDB Entry DOI: 10.7270/Q2TQ60RF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-lactamase (Staphylococcus aureus) | BDBM50079683 (CHEMBL292147 | Sodium; (2S,3R)-3-chloromethyl-6-[1...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 4.32E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Southern Methodist University Curated by ChEMBL | Assay Description Inhibitory activity against serine beta-lactamase, PC1 (class A) derived from Staphylococcus aureus | Bioorg Med Chem Lett 9: 1997-2002 (1999) BindingDB Entry DOI: 10.7270/Q2TQ60RF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-lactamase (Staphylococcus aureus) | BDBM50079681 (CHEMBL60220 | disodium (2S,3S,5R,6Z)-3-[(E)-2-cyan...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 5.04E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Southern Methodist University Curated by ChEMBL | Assay Description Inhibitory activity against serine beta-lactamase, PC1 (class A) derived from Staphylococcus aureus | Bioorg Med Chem Lett 9: 1997-2002 (1999) BindingDB Entry DOI: 10.7270/Q2TQ60RF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-lactamase (Staphylococcus aureus) | BDBM50079688 ((2S,3R,5R,6Z)-3-(chloromethyl)-3-methyl-6-(2-oxido...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 5.27E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Southern Methodist University Curated by ChEMBL | Assay Description Inhibitory activity against serine beta-lactamase, PC1 (class A) derived from Staphylococcus aureus | Bioorg Med Chem Lett 9: 1997-2002 (1999) BindingDB Entry DOI: 10.7270/Q2TQ60RF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-lactamase (Staphylococcus aureus) | BDBM50079686 (CHEMBL292093 | disodium (2S,3R,5R,6Z)-3-methyl-6-(...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 5.79E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Southern Methodist University Curated by ChEMBL | Assay Description Inhibitory activity against serine beta-lactamase, PC1 (class A) derived from Staphylococcus aureus | Bioorg Med Chem Lett 9: 1997-2002 (1999) BindingDB Entry DOI: 10.7270/Q2TQ60RF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-lactamase (Staphylococcus aureus) | BDBM50079693 (CHEMBL59053 | Sodium; (2S,3R)-6-[1-methoxycarbonyl...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 9.40E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Southern Methodist University Curated by ChEMBL | Assay Description Inhibitory activity against serine Beta-lactamase derived from Staphylococcus aureus | Bioorg Med Chem Lett 9: 1997-2002 (1999) BindingDB Entry DOI: 10.7270/Q2TQ60RF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-lactamase (Staphylococcus aureus) | BDBM50079688 ((2S,3R,5R,6Z)-3-(chloromethyl)-3-methyl-6-(2-oxido...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 2.10E+6 | n/a | n/a | n/a | n/a | n/a | n/a |
Southern Methodist University Curated by ChEMBL | Assay Description Inhibitory activity against serine beta-lactamase, PC1 (class A) derived from Staphylococcus aureus | Bioorg Med Chem Lett 9: 1997-2002 (1999) BindingDB Entry DOI: 10.7270/Q2TQ60RF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||