

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Acetylcholinesterase (Rattus norvegicus (rat)) | BDBM8963 (CHEMBL32823 | Homodimeric Tacrine Analog 3b | N-[7...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase CHEMBL MCE MMDB PC cid PC sid PDB UniChem Similars | PubMed | n/a | n/a | 1.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Hong Kong University of Science and Technology Curated by ChEMBL | Assay Description Inhibitory potency against acetylcholinesterase (AChE) of rat cortex homogenate with ethopropazine as BChE inhibitor | Bioorg Med Chem Lett 9: 2335-8 (1999) BindingDB Entry DOI: 10.7270/Q2PK0GPF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Rattus norvegicus (rat)) | BDBM50080157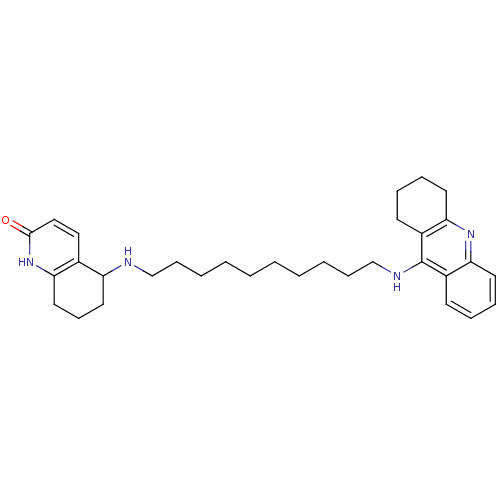 ((RS)-tacrine(10)-hupyridone | 5-[10-(1,2,3,4-Tetra...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid PDB UniChem Similars | PubMed | n/a | n/a | 8.80 | n/a | n/a | n/a | n/a | n/a | n/a |
Hong Kong University of Science and Technology Curated by ChEMBL | Assay Description Inhibitory potency against acetylcholinesterase (AChE) of rat cortex homogenate with ethopropazine as BChE inhibitor | Bioorg Med Chem Lett 9: 2335-8 (1999) BindingDB Entry DOI: 10.7270/Q2PK0GPF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Rattus norvegicus (rat)) | BDBM50080160 (5-[8-(1,2,3,4-Tetrahydro-acridin-9-ylamino)-octyla...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 26 | n/a | n/a | n/a | n/a | n/a | n/a |
Hong Kong University of Science and Technology Curated by ChEMBL | Assay Description Inhibitory potency against acetylcholinesterase (AChE) of rat cortex homogenate with ethopropazine as BChE inhibitor | Bioorg Med Chem Lett 9: 2335-8 (1999) BindingDB Entry DOI: 10.7270/Q2PK0GPF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Rattus norvegicus (rat)) | BDBM50080162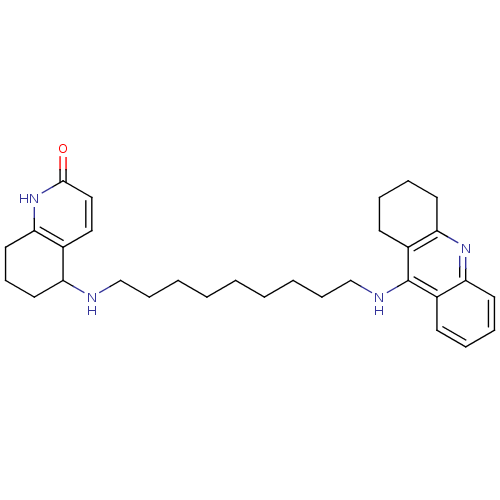 (5-[9-(1,2,3,4-Tetrahydro-acridin-9-ylamino)-nonyla...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 36 | n/a | n/a | n/a | n/a | n/a | n/a |
Hong Kong University of Science and Technology Curated by ChEMBL | Assay Description Inhibitory potency against acetylcholinesterase (AChE) of rat cortex homogenate with ethopropazine as BChE inhibitor | Bioorg Med Chem Lett 9: 2335-8 (1999) BindingDB Entry DOI: 10.7270/Q2PK0GPF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Rattus norvegicus (rat)) | BDBM50366608 (CHEMBL1202834) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 37 | n/a | n/a | n/a | n/a | n/a | n/a |
Hong Kong University of Science and Technology Curated by ChEMBL | Assay Description Inhibitory potency against acetylcholinesterase (AChE) of rat cortex homogenate with ethopropazine as BChE inhibitor | Bioorg Med Chem Lett 9: 2335-8 (1999) BindingDB Entry DOI: 10.7270/Q2PK0GPF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Rattus norvegicus (rat)) | BDBM50366606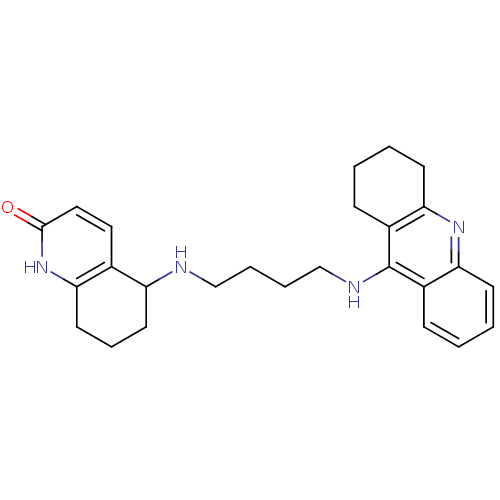 (CHEMBL1202835) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 43 | n/a | n/a | n/a | n/a | n/a | n/a |
Hong Kong University of Science and Technology Curated by ChEMBL | Assay Description Inhibitory potency against acetylcholinesterase (AChE) of rat cortex homogenate with ethopropazine as BChE inhibitor | Bioorg Med Chem Lett 9: 2335-8 (1999) BindingDB Entry DOI: 10.7270/Q2PK0GPF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Rattus norvegicus (rat)) | BDBM50366605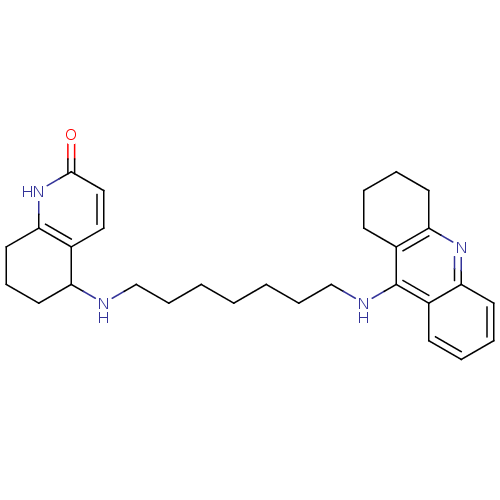 (CHEMBL1202836) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 53 | n/a | n/a | n/a | n/a | n/a | n/a |
Hong Kong University of Science and Technology Curated by ChEMBL | Assay Description Inhibitory potency against acetylcholinesterase (AChE) of rat cortex homogenate with ethopropazine as BChE inhibitor | Bioorg Med Chem Lett 9: 2335-8 (1999) BindingDB Entry DOI: 10.7270/Q2PK0GPF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carboxylic ester hydrolase (Rattus norvegicus (rat)) | BDBM50080157 ((RS)-tacrine(10)-hupyridone | 5-[10-(1,2,3,4-Tetra...) | Reactome pathway KEGG UniProtKB/TrEMBL GoogleScholar AffyNet | CHEMBL PC cid PC sid PDB UniChem Similars | PubMed | n/a | n/a | 82 | n/a | n/a | n/a | n/a | n/a | n/a |
Hong Kong University of Science and Technology Curated by ChEMBL | Assay Description Inhibitory potency against rat serum Butyrylcholinesterase with BW284c51 as AChE inhibitor | Bioorg Med Chem Lett 9: 2335-8 (1999) BindingDB Entry DOI: 10.7270/Q2PK0GPF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carboxylic ester hydrolase (Rattus norvegicus (rat)) | BDBM8961 (1,2,3,4-tetrahydro-9-acridinamine | 1,2,3,4-tetrah...) | Reactome pathway KEGG UniProtKB/TrEMBL GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | PubMed | n/a | n/a | 92 | n/a | n/a | n/a | n/a | n/a | n/a |
Hong Kong University of Science and Technology Curated by ChEMBL | Assay Description Inhibitory potency against rat serum Butyrylcholinesterase with BW284c51 as AChE inhibitor | Bioorg Med Chem Lett 9: 2335-8 (1999) BindingDB Entry DOI: 10.7270/Q2PK0GPF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Rattus norvegicus (rat)) | BDBM50080164 (5-[12-(1,2,3,4-Tetrahydro-acridin-9-ylamino)-dodec...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 112 | n/a | n/a | n/a | n/a | n/a | n/a |
Hong Kong University of Science and Technology Curated by ChEMBL | Assay Description Inhibitory potency against acetylcholinesterase (AChE) of rat cortex homogenate with ethopropazine as BChE inhibitor | Bioorg Med Chem Lett 9: 2335-8 (1999) BindingDB Entry DOI: 10.7270/Q2PK0GPF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Rattus norvegicus (rat)) | BDBM50342601 (CHEMBL1255901 | Huperzine A) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 114 | n/a | n/a | n/a | n/a | n/a | n/a |
Hong Kong University of Science and Technology Curated by ChEMBL | Assay Description Inhibitory potency against acetylcholinesterase (AChE) of rat cortex homogenate with ethopropazine as BChE inhibitor | Bioorg Med Chem Lett 9: 2335-8 (1999) BindingDB Entry DOI: 10.7270/Q2PK0GPF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Rattus norvegicus (rat)) | BDBM50366607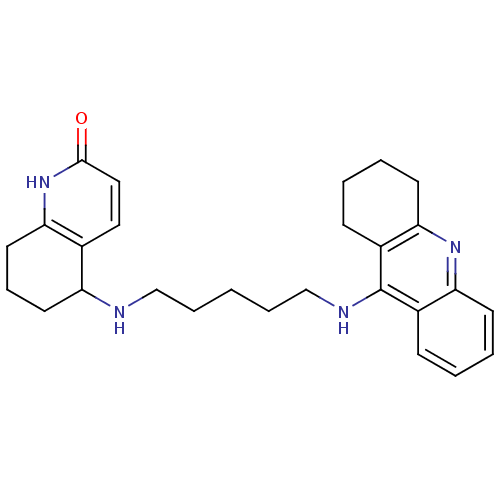 (CHEMBL1202837) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 122 | n/a | n/a | n/a | n/a | n/a | n/a |
Hong Kong University of Science and Technology Curated by ChEMBL | Assay Description Inhibitory potency against acetylcholinesterase (AChE) of rat cortex homogenate with ethopropazine as BChE inhibitor | Bioorg Med Chem Lett 9: 2335-8 (1999) BindingDB Entry DOI: 10.7270/Q2PK0GPF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carboxylic ester hydrolase (Rattus norvegicus (rat)) | BDBM8963 (CHEMBL32823 | Homodimeric Tacrine Analog 3b | N-[7...) | Reactome pathway KEGG UniProtKB/TrEMBL GoogleScholar AffyNet | Purchase CHEMBL MCE MMDB PC cid PC sid PDB UniChem Similars | PubMed | n/a | n/a | 149 | n/a | n/a | n/a | n/a | n/a | n/a |
Hong Kong University of Science and Technology Curated by ChEMBL | Assay Description Inhibitory potency against rat serum Butyrylcholinesterase with BW284c51 as AChE inhibitor | Bioorg Med Chem Lett 9: 2335-8 (1999) BindingDB Entry DOI: 10.7270/Q2PK0GPF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carboxylic ester hydrolase (Rattus norvegicus (rat)) | BDBM50080162 (5-[9-(1,2,3,4-Tetrahydro-acridin-9-ylamino)-nonyla...) | Reactome pathway KEGG UniProtKB/TrEMBL GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 150 | n/a | n/a | n/a | n/a | n/a | n/a |
Hong Kong University of Science and Technology Curated by ChEMBL | Assay Description Inhibitory potency against rat serum Butyrylcholinesterase with BW284c51 as AChE inhibitor | Bioorg Med Chem Lett 9: 2335-8 (1999) BindingDB Entry DOI: 10.7270/Q2PK0GPF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carboxylic ester hydrolase (Rattus norvegicus (rat)) | BDBM50080160 (5-[8-(1,2,3,4-Tetrahydro-acridin-9-ylamino)-octyla...) | Reactome pathway KEGG UniProtKB/TrEMBL GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 166 | n/a | n/a | n/a | n/a | n/a | n/a |
Hong Kong University of Science and Technology Curated by ChEMBL | Assay Description Inhibitory potency against rat serum Butyrylcholinesterase with BW284c51 as AChE inhibitor | Bioorg Med Chem Lett 9: 2335-8 (1999) BindingDB Entry DOI: 10.7270/Q2PK0GPF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carboxylic ester hydrolase (Rattus norvegicus (rat)) | BDBM50366607 (CHEMBL1202837) | Reactome pathway KEGG UniProtKB/TrEMBL GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 220 | n/a | n/a | n/a | n/a | n/a | n/a |
Hong Kong University of Science and Technology Curated by ChEMBL | Assay Description Inhibitory potency against rat serum Butyrylcholinesterase with BW284c51 as AChE inhibitor | Bioorg Med Chem Lett 9: 2335-8 (1999) BindingDB Entry DOI: 10.7270/Q2PK0GPF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Rattus norvegicus (rat)) | BDBM8961 (1,2,3,4-tetrahydro-9-acridinamine | 1,2,3,4-tetrah...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | PDB PubMed | n/a | n/a | 223 | n/a | n/a | n/a | n/a | n/a | n/a |
Hong Kong University of Science and Technology Curated by ChEMBL | Assay Description Inhibitory potency against acetylcholinesterase (AChE) of rat cortex homogenate with ethopropazine as BChE inhibitor | Bioorg Med Chem Lett 9: 2335-8 (1999) BindingDB Entry DOI: 10.7270/Q2PK0GPF | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Carboxylic ester hydrolase (Rattus norvegicus (rat)) | BDBM50366608 (CHEMBL1202834) | Reactome pathway KEGG UniProtKB/TrEMBL GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 223 | n/a | n/a | n/a | n/a | n/a | n/a |
Hong Kong University of Science and Technology Curated by ChEMBL | Assay Description Inhibitory potency against rat serum Butyrylcholinesterase with BW284c51 as AChE inhibitor | Bioorg Med Chem Lett 9: 2335-8 (1999) BindingDB Entry DOI: 10.7270/Q2PK0GPF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carboxylic ester hydrolase (Rattus norvegicus (rat)) | BDBM50366606 (CHEMBL1202835) | Reactome pathway KEGG UniProtKB/TrEMBL GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 228 | n/a | n/a | n/a | n/a | n/a | n/a |
Hong Kong University of Science and Technology Curated by ChEMBL | Assay Description Inhibitory potency against rat serum Butyrylcholinesterase with BW284c51 as AChE inhibitor | Bioorg Med Chem Lett 9: 2335-8 (1999) BindingDB Entry DOI: 10.7270/Q2PK0GPF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carboxylic ester hydrolase (Rattus norvegicus (rat)) | BDBM50366605 (CHEMBL1202836) | Reactome pathway KEGG UniProtKB/TrEMBL GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 233 | n/a | n/a | n/a | n/a | n/a | n/a |
Hong Kong University of Science and Technology Curated by ChEMBL | Assay Description Inhibitory potency against rat serum Butyrylcholinesterase with BW284c51 as AChE inhibitor | Bioorg Med Chem Lett 9: 2335-8 (1999) BindingDB Entry DOI: 10.7270/Q2PK0GPF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carboxylic ester hydrolase (Rattus norvegicus (rat)) | BDBM50080164 (5-[12-(1,2,3,4-Tetrahydro-acridin-9-ylamino)-dodec...) | Reactome pathway KEGG UniProtKB/TrEMBL GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 434 | n/a | n/a | n/a | n/a | n/a | n/a |
Hong Kong University of Science and Technology Curated by ChEMBL | Assay Description Inhibitory potency against rat serum Butyrylcholinesterase with BW284c51 as AChE inhibitor | Bioorg Med Chem Lett 9: 2335-8 (1999) BindingDB Entry DOI: 10.7270/Q2PK0GPF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carboxylic ester hydrolase (Rattus norvegicus (rat)) | BDBM50342601 (CHEMBL1255901 | Huperzine A) | Reactome pathway KEGG UniProtKB/TrEMBL GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 1.35E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Hong Kong University of Science and Technology Curated by ChEMBL | Assay Description Inhibitory potency against rat serum Butyrylcholinesterase with BW284c51 as AChE inhibitor | Bioorg Med Chem Lett 9: 2335-8 (1999) BindingDB Entry DOI: 10.7270/Q2PK0GPF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||