

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Arginine--tRNA ligase, cytoplasmic (Homo sapiens (Human)) | BDBM50091515 (Aminoalkyl adenylate and aminoacyl sulfamate analo...) | PDB Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 4.5 | n/a | n/a | n/a | n/a | n/a | n/a |
SmithKline Beecham Pharmaceuticals Curated by ChEMBL | Assay Description Inhibitory activity against cognate Staphylococcus aureus Arginyl-tRNA synthetase | Bioorg Med Chem Lett 10: 1871-4 (2000) BindingDB Entry DOI: 10.7270/Q2TX3FWJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Arginine--tRNA ligase, cytoplasmic (Homo sapiens (Human)) | BDBM50091518 (CHEMBL434782 | Phosphoric acid (S)-2-amino-5-guani...) | PDB Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 7.5 | n/a | n/a | n/a | n/a | n/a | n/a |
SmithKline Beecham Pharmaceuticals Curated by ChEMBL | Assay Description Inhibitory activity against cognate Staphylococcus aureus Arginyl-tRNA synthetase | Bioorg Med Chem Lett 10: 1871-4 (2000) BindingDB Entry DOI: 10.7270/Q2TX3FWJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Threonine--tRNA ligase 1, cytoplasmic (Homo sapiens (Human)) | BDBM50366646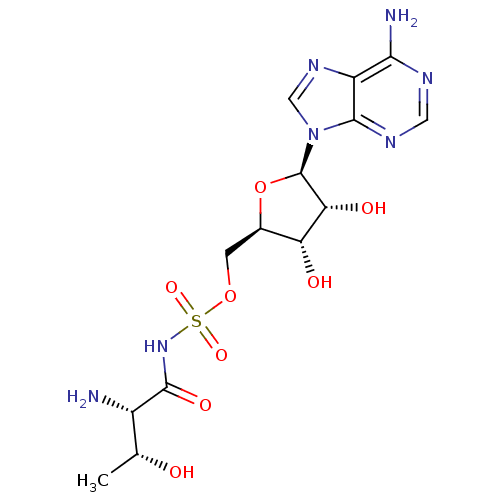 (CHEMBL1163068) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL MMDB PC cid PC sid PDB UniChem Similars | PubMed | n/a | n/a | 15 | n/a | n/a | n/a | n/a | n/a | n/a |
SmithKline Beecham Pharmaceuticals Curated by ChEMBL | Assay Description Inhibitory activity against cognate Staphylococcus aureus threonyl tRNA synthetase | Bioorg Med Chem Lett 10: 1871-4 (2000) BindingDB Entry DOI: 10.7270/Q2TX3FWJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histidine--tRNA ligase, cytoplasmic (Homo sapiens (Human)) | BDBM50091516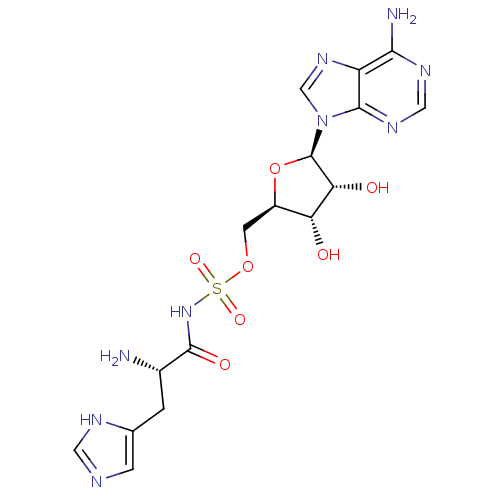 (5'-O-[(L-HISTIDYLAMINO)SULFONYL]ADENOSINE | CHEMBL...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL MMDB PC cid PC sid PDB UniChem Similars | PubMed | n/a | n/a | 130 | n/a | n/a | n/a | n/a | n/a | n/a |
SmithKline Beecham Pharmaceuticals Curated by ChEMBL | Assay Description Inhibitory activity against cognate Staphylococcus aureus Histidyl-tRNA synthetase | Bioorg Med Chem Lett 10: 1871-4 (2000) BindingDB Entry DOI: 10.7270/Q2TX3FWJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||