

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Muscarinic acetylcholine receptor M2 (GUINEA PIG) | BDBM50421416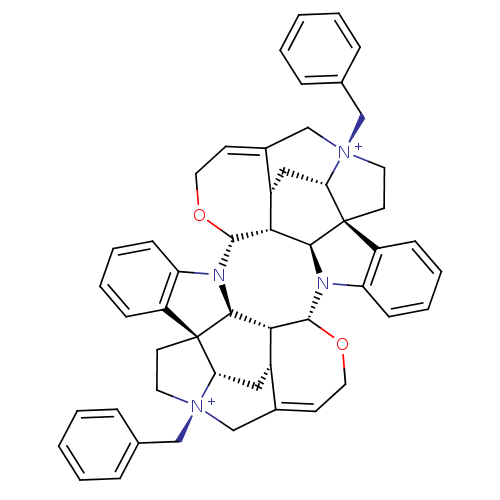 (CHEMBL2311018) | PDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | n/a | n/a | 69 | n/a | n/a | n/a | n/a |
University of Würzburg Curated by ChEMBL | Assay Description Allosteric inhibition of [3H]-NMS (N-methylscopolamine) dissociation from porcine cardiac Muscarinic acetylcholine receptor M2 | Bioorg Med Chem Lett 10: 2529-32 (2001) BindingDB Entry DOI: 10.7270/Q2G1602G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Muscarinic acetylcholine receptor M2 (GUINEA PIG) | BDBM50093889 (CHEMBL407071 | Caracurine V derivative) | PDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | n/a | n/a | 507 | n/a | n/a | n/a | n/a |
University of Würzburg Curated by ChEMBL | Assay Description Allosteric inhibition of [3H]-NMS (N-methylscopolamine) dissociation from porcine cardiac Muscarinic acetylcholine receptor M2 | Bioorg Med Chem Lett 10: 2529-32 (2001) BindingDB Entry DOI: 10.7270/Q2G1602G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Muscarinic acetylcholine receptor M2 (GUINEA PIG) | BDBM50093894 (CHEMBL385915 | Caracurine V derivative) | PDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | n/a | n/a | 376 | n/a | n/a | n/a | n/a |
University of Würzburg Curated by ChEMBL | Assay Description Allosteric inhibition of [3H]-NMS (N-methylscopolamine) dissociation from porcine cardiac Muscarinic acetylcholine receptor M2 | Bioorg Med Chem Lett 10: 2529-32 (2001) BindingDB Entry DOI: 10.7270/Q2G1602G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Muscarinic acetylcholine receptor M2 (GUINEA PIG) | BDBM50093898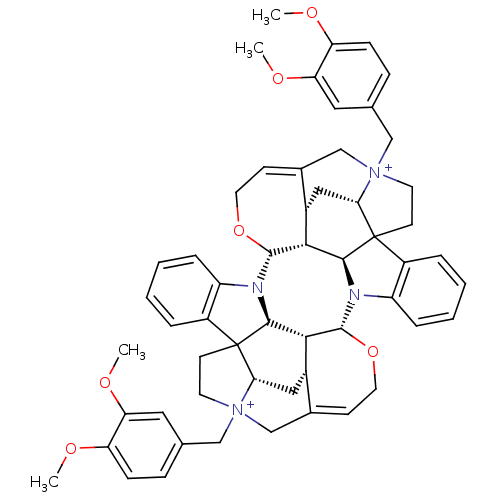 (CHEMBL412718 | Caracurine V derivative) | PDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | n/a | n/a | 342 | n/a | n/a | n/a | n/a |
University of Würzburg Curated by ChEMBL | Assay Description Allosteric inhibition of [3H]-NMS (N-methylscopolamine) dissociation from porcine cardiac Muscarinic acetylcholine receptor M2 | Bioorg Med Chem Lett 10: 2529-32 (2001) BindingDB Entry DOI: 10.7270/Q2G1602G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Muscarinic acetylcholine receptor M2 (GUINEA PIG) | BDBM50093892 (CHEMBL312920 | Caracurine V derivative) | PDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | n/a | n/a | 1.19E+3 | n/a | n/a | n/a | n/a |
University of Würzburg Curated by ChEMBL | Assay Description Allosteric inhibition of [3H]-NMS (N-methylscopolamine) dissociation from porcine cardiac Muscarinic acetylcholine receptor M2 | Bioorg Med Chem Lett 10: 2529-32 (2001) BindingDB Entry DOI: 10.7270/Q2G1602G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Muscarinic acetylcholine receptor M2 (GUINEA PIG) | BDBM50093899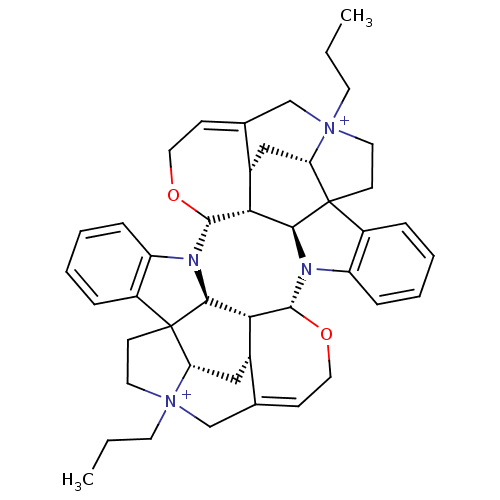 (CHEMBL314491 | Caracurine V derivative) | PDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | n/a | n/a | 30 | n/a | n/a | n/a | n/a |
University of Würzburg Curated by ChEMBL | Assay Description Allosteric inhibition of [3H]-NMS (N-methylscopolamine) dissociation from porcine cardiac Muscarinic acetylcholine receptor M2 | Bioorg Med Chem Lett 10: 2529-32 (2001) BindingDB Entry DOI: 10.7270/Q2G1602G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Muscarinic acetylcholine receptor M2 (GUINEA PIG) | BDBM50093895 (CHEMBL407440 | Caracurine V derivative) | PDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | n/a | n/a | 1.56E+3 | n/a | n/a | n/a | n/a |
University of Würzburg Curated by ChEMBL | Assay Description Allosteric inhibition of [3H]-NMS (N-methylscopolamine) dissociation from porcine cardiac Muscarinic acetylcholine receptor M2 | Bioorg Med Chem Lett 10: 2529-32 (2001) BindingDB Entry DOI: 10.7270/Q2G1602G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Muscarinic acetylcholine receptor M2 (GUINEA PIG) | BDBM50093890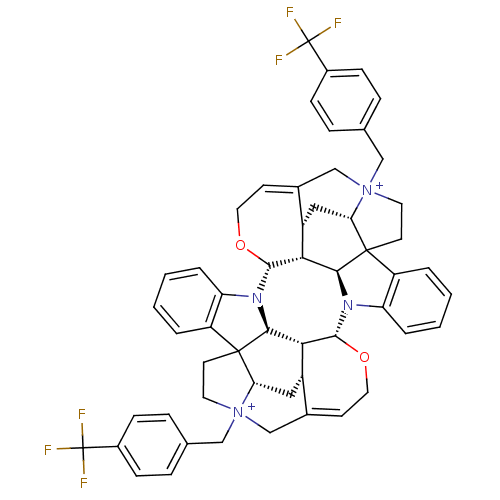 (CHEMBL412670 | Caracurine V derivative) | PDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | n/a | n/a | 1.75E+3 | n/a | n/a | n/a | n/a |
University of Würzburg Curated by ChEMBL | Assay Description Allosteric inhibition of [3H]-NMS (N-methylscopolamine) dissociation from porcine cardiac Muscarinic acetylcholine receptor M2 | Bioorg Med Chem Lett 10: 2529-32 (2001) BindingDB Entry DOI: 10.7270/Q2G1602G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Muscarinic acetylcholine receptor M2 (GUINEA PIG) | BDBM50093896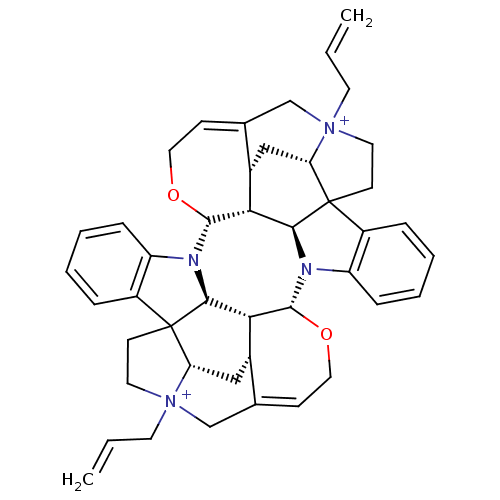 (CHEMBL311649 | Caracurine V derivative) | PDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | n/a | n/a | 3 | n/a | n/a | n/a | n/a |
University of Würzburg Curated by ChEMBL | Assay Description Allosteric inhibition of [3H]-NMS (N-methylscopolamine) dissociation from porcine cardiac Muscarinic acetylcholine receptor M2 | Bioorg Med Chem Lett 10: 2529-32 (2001) BindingDB Entry DOI: 10.7270/Q2G1602G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Muscarinic acetylcholine receptor M2 (GUINEA PIG) | BDBM50093891 (CHEMBL315340 | Caracurine V derivative) | PDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | n/a | n/a | 8 | n/a | n/a | n/a | n/a |
University of Würzburg Curated by ChEMBL | Assay Description Allosteric inhibition of [3H]-NMS (N-methylscopolamine) dissociation from porcine cardiac Muscarinic acetylcholine receptor M2 | Bioorg Med Chem Lett 10: 2529-32 (2001) BindingDB Entry DOI: 10.7270/Q2G1602G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Muscarinic acetylcholine receptor M2 (GUINEA PIG) | BDBM50093893 (CHEMBL433343 | Caracurine V derivative) | PDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | n/a | n/a | 4 | n/a | n/a | n/a | n/a |
University of Würzburg Curated by ChEMBL | Assay Description Allosteric inhibition of [3H]-NMS (N-methylscopolamine) dissociation from porcine cardiac Muscarinic acetylcholine receptor M2 | Bioorg Med Chem Lett 10: 2529-32 (2001) BindingDB Entry DOI: 10.7270/Q2G1602G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Muscarinic acetylcholine receptor M2 (GUINEA PIG) | BDBM50093888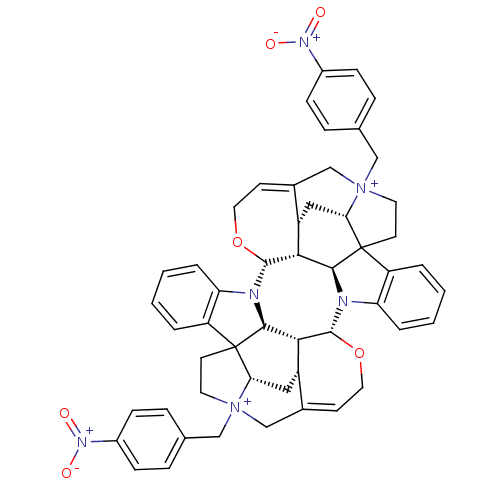 (CHEMBL405691 | Caracurine V derivative) | PDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | n/a | n/a | 315 | n/a | n/a | n/a | n/a |
University of Würzburg Curated by ChEMBL | Assay Description Allosteric inhibition of [3H]-NMS (N-methylscopolamine) dissociation from porcine cardiac Muscarinic acetylcholine receptor M2 | Bioorg Med Chem Lett 10: 2529-32 (2001) BindingDB Entry DOI: 10.7270/Q2G1602G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||