 Found 15 hits of Enzyme Inhibition Constant Data
Found 15 hits of Enzyme Inhibition Constant Data Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
17-beta-hydroxysteroid dehydrogenase type 3
(Homo sapiens (Human)) | BDBM85469
(Diphenyl-p-benzoquinone, 6)Show InChI InChI=1S/C14H12O2/c1-2-10-8-14(16)12(9-13(10)15)11-6-4-3-5-7-11/h2-9,15-16H,1H2 | Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2.70E+3 | n/a | n/a | n/a | n/a | 7.45 | 37 |
Cardiff University
| Assay Description
Inhibition assay of human testes mcrosomal 17 beta-hydroxysteroid dehydrogenase for the reduction of androstenedione. |
J Enzym Inhib 16: 35-45 (2001)
Article DOI: 10.1080/14756360109162353
BindingDB Entry DOI: 10.7270/Q2SX6BRB |
More data for this
Ligand-Target Pair | |
17-beta-hydroxysteroid dehydrogenase type 3
(Homo sapiens (Human)) | BDBM24781
(2-phenyl-1,4-benzoquinone, 10 | 2-phenylcyclohexa-...)Show InChI InChI=1S/C12H8O2/c13-10-6-7-12(14)11(8-10)9-4-2-1-3-5-9/h1-8H | Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 5.70E+3 | n/a | n/a | n/a | n/a | 7.45 | 37 |
Cardiff University
| Assay Description
Inhibition assay of human testes mcrosomal 17 beta-hydroxysteroid dehydrogenase for the reduction of androstenedione. |
J Enzym Inhib 16: 35-45 (2001)
Article DOI: 10.1080/14756360109162353
BindingDB Entry DOI: 10.7270/Q2SX6BRB |
More data for this
Ligand-Target Pair | |
17-beta-hydroxysteroid dehydrogenase type 3
(Homo sapiens (Human)) | BDBM26664
(7-Hydroxy-flavone, 5a | 7-Hydroxyflavone, 11 | 7-h...)Show InChI InChI=1S/C15H10O3/c16-11-6-7-12-13(17)9-14(18-15(12)8-11)10-4-2-1-3-5-10/h1-9,16H | Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
KEGG
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 9.00E+3 | n/a | n/a | n/a | n/a | 7.45 | 37 |
Cardiff University
| Assay Description
Inhibition assay of human testes mcrosomal 17 beta-hydroxysteroid dehydrogenase for the reduction of androstenedione. |
J Enzym Inhib 16: 35-45 (2001)
Article DOI: 10.1080/14756360109162353
BindingDB Entry DOI: 10.7270/Q2SX6BRB |
More data for this
Ligand-Target Pair | |
17-beta-hydroxysteroid dehydrogenase type 3
(Homo sapiens (Human)) | BDBM85468
(17Beta-HSD Inhibitor, 3)Show SMILES O=[#6](-[#8]\[#6](=[#6](/c1ccccc1)-c1ccccc1)-c1ccccc1)C1([#6]C#C)[#6]-[#6](=O)-[#7]-[#6]1=O Show InChI InChI=1S/C28H21NO4/c1-2-18-28(19-23(30)29-26(28)31)27(32)33-25(22-16-10-5-11-17-22)24(20-12-6-3-7-13-20)21-14-8-4-9-15-21/h1,3-17H,18-19H2,(H,29,30,31) | Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 9.15E+3 | n/a | n/a | n/a | n/a | 7.45 | 37 |
Cardiff University
| Assay Description
Inhibition assay of human testes mcrosomal 17 beta-hydroxysteroid dehydrogenase for the reduction of androstenedione. |
J Enzym Inhib 16: 35-45 (2001)
Article DOI: 10.1080/14756360109162353
BindingDB Entry DOI: 10.7270/Q2SX6BRB |
More data for this
Ligand-Target Pair | |
17-beta-hydroxysteroid dehydrogenase type 3
(Homo sapiens (Human)) | BDBM50009001

(5,6,7-Trihydroxyflavone | 5,6,7-trihydroxy-2-pheny...)Show InChI InChI=1S/C15H10O5/c16-9-6-11(8-4-2-1-3-5-8)20-12-7-10(17)14(18)15(19)13(9)12/h1-7,17-19H | Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
KEGG
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 9.30E+3 | n/a | n/a | n/a | n/a | 7.45 | 37 |
Cardiff University
| Assay Description
Inhibition assay of human testes mcrosomal 17 beta-hydroxysteroid dehydrogenase for the reduction of androstenedione. |
J Enzym Inhib 16: 35-45 (2001)
Article DOI: 10.1080/14756360109162353
BindingDB Entry DOI: 10.7270/Q2SX6BRB |
More data for this
Ligand-Target Pair | |
17-beta-hydroxysteroid dehydrogenase type 3
(Homo sapiens (Human)) | BDBM9461
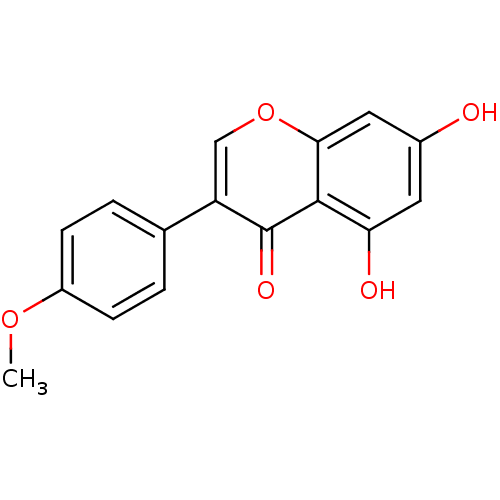
(5,7-dihydroxy-3-(4-methoxyphenyl)-4H-chromen-4-one...)Show InChI InChI=1S/C16H12O5/c1-20-11-4-2-9(3-5-11)12-8-21-14-7-10(17)6-13(18)15(14)16(12)19/h2-8,17-18H,1H3 | Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 1.08E+4 | n/a | n/a | n/a | n/a | 7.45 | 37 |
Cardiff University
| Assay Description
Inhibition assay of human testes mcrosomal 17 beta-hydroxysteroid dehydrogenase for the reduction of androstenedione. |
J Enzym Inhib 16: 35-45 (2001)
Article DOI: 10.1080/14756360109162353
BindingDB Entry DOI: 10.7270/Q2SX6BRB |
More data for this
Ligand-Target Pair | |
17-beta-hydroxysteroid dehydrogenase type 3
(Homo sapiens (Human)) | BDBM34125

(2-Phenylhydroquinone, 7 | hydroquinone derivative,...)Show InChI InChI=1S/C12H10O2/c13-10-6-7-12(14)11(8-10)9-4-2-1-3-5-9/h1-8,13-14H | Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 1.48E+4 | n/a | n/a | n/a | n/a | 7.45 | 37 |
Cardiff University
| Assay Description
Inhibition assay of human testes mcrosomal 17 beta-hydroxysteroid dehydrogenase for the reduction of androstenedione. |
J Enzym Inhib 16: 35-45 (2001)
Article DOI: 10.1080/14756360109162353
BindingDB Entry DOI: 10.7270/Q2SX6BRB |
More data for this
Ligand-Target Pair | |
17-beta-hydroxysteroid dehydrogenase type 3
(Homo sapiens (Human)) | BDBM7458

(5,7-dihydroxy-2-(4-hydroxyphenyl)-4H-chromen-4-one...)Show InChI InChI=1S/C15H10O5/c16-9-3-1-8(2-4-9)13-7-12(19)15-11(18)5-10(17)6-14(15)20-13/h1-7,16-18H | Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 1.64E+4 | n/a | n/a | n/a | n/a | 7.45 | 37 |
Cardiff University
| Assay Description
Inhibition assay of human testes mcrosomal 17 beta-hydroxysteroid dehydrogenase for the reduction of androstenedione. |
J Enzym Inhib 16: 35-45 (2001)
Article DOI: 10.1080/14756360109162353
BindingDB Entry DOI: 10.7270/Q2SX6BRB |
More data for this
Ligand-Target Pair | |
17-beta-hydroxysteroid dehydrogenase type 3
(Homo sapiens (Human)) | BDBM50081950
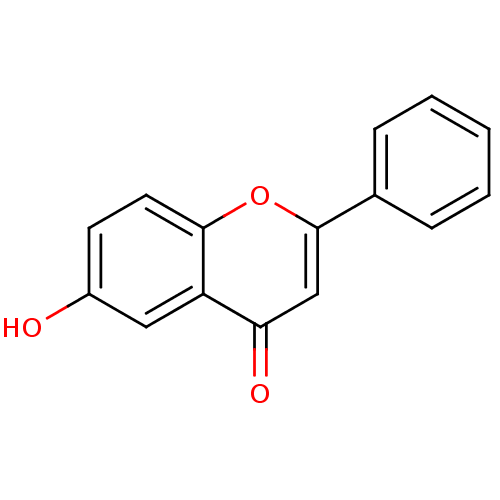
(6-Hydroxy-2-phenyl-chromen-4-one | 6-Hydroxyflavon...)Show InChI InChI=1S/C15H10O3/c16-11-6-7-14-12(8-11)13(17)9-15(18-14)10-4-2-1-3-5-10/h1-9,16H | Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
KEGG
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 1.64E+4 | n/a | n/a | n/a | n/a | 7.45 | 37 |
Cardiff University
| Assay Description
Inhibition assay of human testes mcrosomal 17 beta-hydroxysteroid dehydrogenase for the reduction of androstenedione. |
J Enzym Inhib 16: 35-45 (2001)
Article DOI: 10.1080/14756360109162353
BindingDB Entry DOI: 10.7270/Q2SX6BRB |
More data for this
Ligand-Target Pair | |
17-beta-hydroxysteroid dehydrogenase type 3
(Homo sapiens (Human)) | BDBM7458

(5,7-dihydroxy-2-(4-hydroxyphenyl)-4H-chromen-4-one...)Show InChI InChI=1S/C15H10O5/c16-9-3-1-8(2-4-9)13-7-12(19)15-11(18)5-10(17)6-14(15)20-13/h1-7,16-18H | Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 2.13E+4 | n/a | n/a | n/a | n/a | 7.45 | 37 |
Cardiff University
| Assay Description
Inhibition assay of human testes mcrosomal 17 beta-hydroxysteroid dehydrogenase for the reduction of androstenedione. |
J Enzym Inhib 16: 35-45 (2001)
Article DOI: 10.1080/14756360109162353
BindingDB Entry DOI: 10.7270/Q2SX6BRB |
More data for this
Ligand-Target Pair | |
17-beta-hydroxysteroid dehydrogenase type 3
(Homo sapiens (Human)) | BDBM19459

(5,7-dihydroxy-3-(4-hydroxyphenyl)-4H-chromen-4-one...)Show InChI InChI=1S/C15H10O5/c16-9-3-1-8(2-4-9)11-7-20-13-6-10(17)5-12(18)14(13)15(11)19/h1-7,16-18H | Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 3.03E+4 | n/a | n/a | n/a | n/a | 7.45 | 37 |
Cardiff University
| Assay Description
Inhibition assay of human testes mcrosomal 17 beta-hydroxysteroid dehydrogenase for the reduction of androstenedione. |
J Enzym Inhib 16: 35-45 (2001)
Article DOI: 10.1080/14756360109162353
BindingDB Entry DOI: 10.7270/Q2SX6BRB |
More data for this
Ligand-Target Pair | |
17-beta-hydroxysteroid dehydrogenase type 3
(Homo sapiens (Human)) | BDBM7457
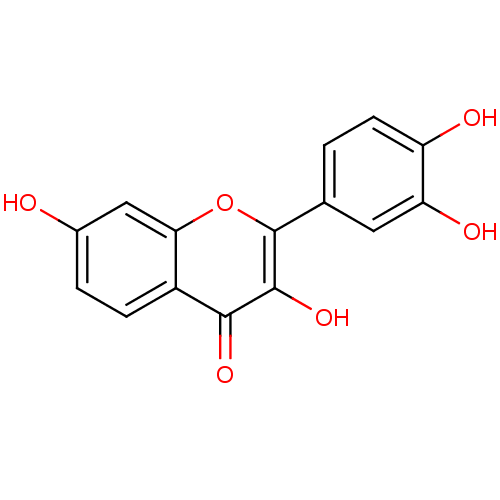
(2-(3,4-dihydroxyphenyl)-3,7-dihydroxy-4H-chromen-4...)Show InChI InChI=1S/C15H10O6/c16-8-2-3-9-12(6-8)21-15(14(20)13(9)19)7-1-4-10(17)11(18)5-7/h1-6,16-18,20H | Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 3.24E+4 | n/a | n/a | n/a | n/a | 7.45 | 37 |
Cardiff University
| Assay Description
Inhibition assay of human testes mcrosomal 17 beta-hydroxysteroid dehydrogenase for the reduction of androstenedione. |
J Enzym Inhib 16: 35-45 (2001)
Article DOI: 10.1080/14756360109162353
BindingDB Entry DOI: 10.7270/Q2SX6BRB |
More data for this
Ligand-Target Pair | |
17-beta-hydroxysteroid dehydrogenase type 3
(Homo sapiens (Human)) | BDBM71545
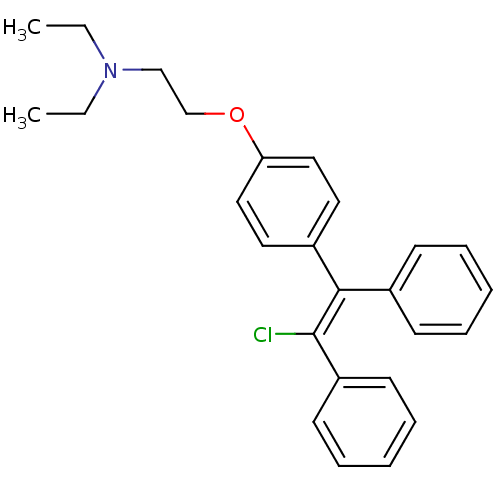
(2-[4-[(Z)-2-chloranyl-1,2-diphenyl-ethenyl]phenoxy...)Show SMILES CCN(CC)CCOc1ccc(cc1)C(=C(/Cl)c1ccccc1)\c1ccccc1 Show InChI InChI=1S/C26H28ClNO/c1-3-28(4-2)19-20-29-24-17-15-22(16-18-24)25(21-11-7-5-8-12-21)26(27)23-13-9-6-10-14-23/h5-18H,3-4,19-20H2,1-2H3/b26-25- | Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
KEGG
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 7.62E+4 | n/a | n/a | n/a | n/a | 7.45 | 37 |
Cardiff University
| Assay Description
Inhibition assay of human testes mcrosomal 17 beta-hydroxysteroid dehydrogenase for the reduction of androstenedione. |
J Enzym Inhib 16: 35-45 (2001)
Article DOI: 10.1080/14756360109162353
BindingDB Entry DOI: 10.7270/Q2SX6BRB |
More data for this
Ligand-Target Pair | |
17-beta-hydroxysteroid dehydrogenase type 3
(Homo sapiens (Human)) | BDBM85467
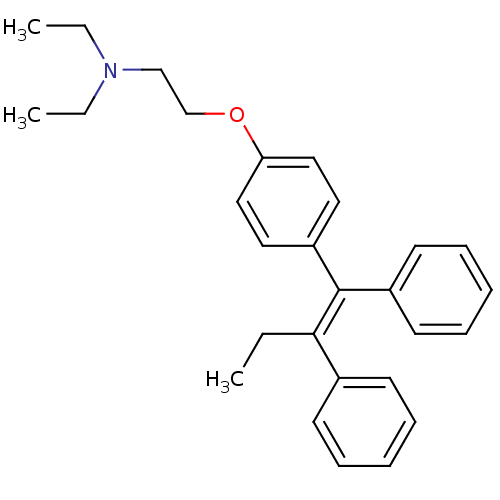
(Tamoxifen, 1)Show SMILES CCN(CC)CCOc1ccc(cc1)C(=C(/CC)c1ccccc1)\c1ccccc1 Show InChI InChI=1S/C28H33NO/c1-4-27(23-13-9-7-10-14-23)28(24-15-11-8-12-16-24)25-17-19-26(20-18-25)30-22-21-29(5-2)6-3/h7-20H,4-6,21-22H2,1-3H3/b28-27+ | Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 9.81E+4 | n/a | n/a | n/a | n/a | 7.45 | 37 |
Cardiff University
| Assay Description
Inhibition assay of human testes mcrosomal 17 beta-hydroxysteroid dehydrogenase for the reduction of androstenedione. |
J Enzym Inhib 16: 35-45 (2001)
Article DOI: 10.1080/14756360109162353
BindingDB Entry DOI: 10.7270/Q2SX6BRB |
More data for this
Ligand-Target Pair | |
17-beta-hydroxysteroid dehydrogenase type 3
(Homo sapiens (Human)) | BDBM15236

(3,5,7-trihydroxy-2-(3,4,5-trihydroxyphenyl)-4H-chr...)Show SMILES Oc1cc(O)c2c(c1)oc(-c1cc(O)c(O)c(O)c1)c(O)c2=O Show InChI InChI=1S/C15H10O8/c16-6-3-7(17)11-10(4-6)23-15(14(22)13(11)21)5-1-8(18)12(20)9(19)2-5/h1-4,16-20,22H | Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 1.01E+5 | n/a | n/a | n/a | n/a | 7.45 | 37 |
Cardiff University
| Assay Description
Inhibition assay of human testes mcrosomal 17 beta-hydroxysteroid dehydrogenase for the reduction of androstenedione. |
J Enzym Inhib 16: 35-45 (2001)
Article DOI: 10.1080/14756360109162353
BindingDB Entry DOI: 10.7270/Q2SX6BRB |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data