 Found 22 hits of Enzyme Inhibition Constant Data
Found 22 hits of Enzyme Inhibition Constant Data Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Acetylcholinesterase
(Homo sapiens (Human)) | BDBM8960

((+/-)-2-[(1-benzylpiperidin-4-yl)methyl]-5,6-dimet...)Show SMILES COc1cc2CC(CC3CCN(Cc4ccccc4)CC3)C(=O)c2cc1OC Show InChI InChI=1S/C24H29NO3/c1-27-22-14-19-13-20(24(26)21(19)15-23(22)28-2)12-17-8-10-25(11-9-17)16-18-6-4-3-5-7-18/h3-7,14-15,17,20H,8-13,16H2,1-2H3 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
PC cid
PC sid
PDB
UniChem
Patents
Similars
| DrugBank
PDB
PubMed
| n/a | n/a | 40 | n/a | n/a | n/a | n/a | n/a | n/a |
Università di Bologna
Curated by ChEMBL
| Assay Description
Inhibitory concentration required against acetylcholinesterase activity |
J Med Chem 44: 4011-4 (2001)
BindingDB Entry DOI: 10.7270/Q298869Q |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Acetylcholinesterase
(Homo sapiens (Human)) | BDBM8961

(1,2,3,4-tetrahydro-9-acridinamine | 1,2,3,4-tetrah...)Show InChI InChI=1S/C13H14N2/c14-13-9-5-1-3-7-11(9)15-12-8-4-2-6-10(12)13/h1,3,5,7H,2,4,6,8H2,(H2,14,15) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
PubMed
| n/a | n/a | 180 | n/a | n/a | n/a | n/a | n/a | n/a |
Università di Bologna
Curated by ChEMBL
| Assay Description
Inhibitory concentration required against acetylcholinesterase activity |
J Med Chem 44: 4011-4 (2001)
BindingDB Entry DOI: 10.7270/Q298869Q |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Acetylcholinesterase
(Homo sapiens (Human)) | BDBM50106465
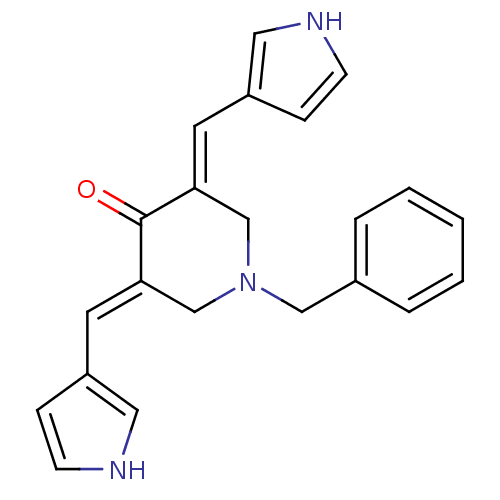
(1-Benzyl-3,5-bis-(1H-pyrrol-3-ylmethylene)-piperid...)Show SMILES O=C1\C(CN(Cc2ccccc2)C\C1=C/c1cc[nH]c1)=C\c1cc[nH]c1 Show InChI InChI=1S/C22H21N3O/c26-22-20(10-18-6-8-23-12-18)15-25(14-17-4-2-1-3-5-17)16-21(22)11-19-7-9-24-13-19/h1-13,23-24H,14-16H2/b20-10+,21-11+ | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 6.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Università di Bologna
Curated by ChEMBL
| Assay Description
Inhibitory concentration required against acetylcholinesterase activity |
J Med Chem 44: 4011-4 (2001)
BindingDB Entry DOI: 10.7270/Q298869Q |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Homo sapiens (Human)) | BDBM50106462
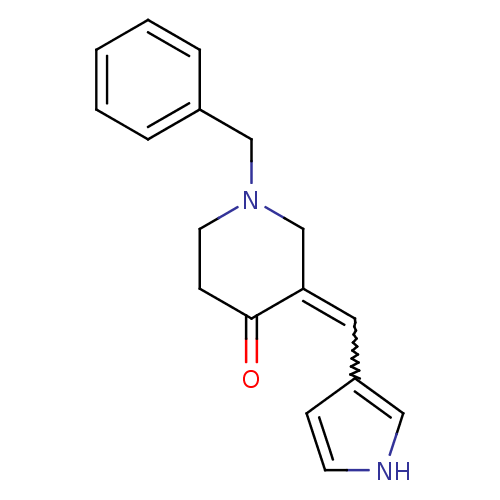
(1-Benzyl-3-(1H-pyrrol-3-ylmethylene)-piperidin-4-o...)Show SMILES O=C1CCN(Cc2ccccc2)CC1=Cc1cc[nH]c1 |w:14.16| Show InChI InChI=1S/C17H18N2O/c20-17-7-9-19(12-14-4-2-1-3-5-14)13-16(17)10-15-6-8-18-11-15/h1-6,8,10-11,18H,7,9,12-13H2 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 6.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Università di Bologna
Curated by ChEMBL
| Assay Description
Inhibitory concentration required against acetylcholinesterase activity |
J Med Chem 44: 4011-4 (2001)
BindingDB Entry DOI: 10.7270/Q298869Q |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Homo sapiens (Human)) | BDBM50106457
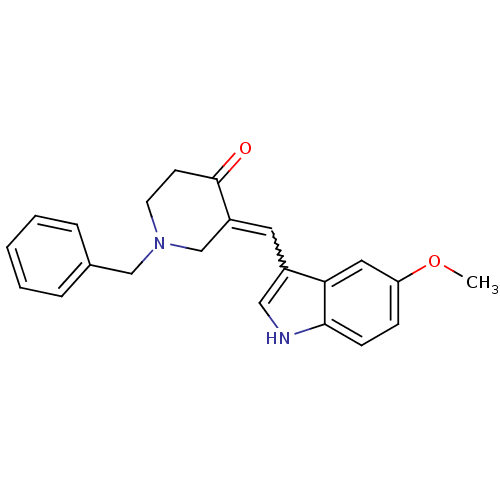
(3-(1-Benzoyl-1H-pyrrol-3-ylmethylene)-1-benzyl-pip...)Show SMILES COc1ccc2[nH]cc(C=C3CN(Cc4ccccc4)CCC3=O)c2c1 |w:9.8| Show InChI InChI=1S/C22H22N2O2/c1-26-19-7-8-21-20(12-19)17(13-23-21)11-18-15-24(10-9-22(18)25)14-16-5-3-2-4-6-16/h2-8,11-13,23H,9-10,14-15H2,1H3 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 6.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Università di Bologna
Curated by ChEMBL
| Assay Description
Inhibitory concentration required against acetylcholinesterase activity |
J Med Chem 44: 4011-4 (2001)
BindingDB Entry DOI: 10.7270/Q298869Q |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Homo sapiens (Human)) | BDBM50106455
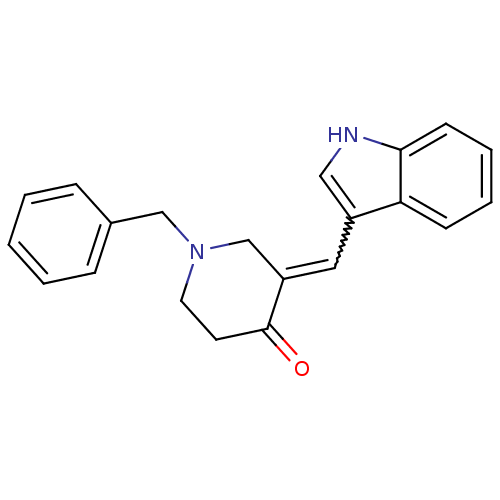
(3-(1-Benzoyl-1H-pyrrol-3-ylmethylene)-1-benzyl-pip...)Show SMILES O=C1CCN(Cc2ccccc2)CC1=Cc1c[nH]c2ccccc12 |w:14.16| Show InChI InChI=1S/C21H20N2O/c24-21-10-11-23(14-16-6-2-1-3-7-16)15-18(21)12-17-13-22-20-9-5-4-8-19(17)20/h1-9,12-13,22H,10-11,14-15H2 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 8.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Università di Bologna
Curated by ChEMBL
| Assay Description
Inhibitory concentration required against acetylcholinesterase activity |
J Med Chem 44: 4011-4 (2001)
BindingDB Entry DOI: 10.7270/Q298869Q |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Homo sapiens (Human)) | BDBM50106454

(1-Benzyl-3-(1H-pyrrol-2-ylmethylene)-piperidin-4-o...)Show SMILES O=C1CCN(Cc2ccccc2)CC1=Cc1ccc[nH]1 |w:14.16| Show InChI InChI=1S/C17H18N2O/c20-17-8-10-19(12-14-5-2-1-3-6-14)13-15(17)11-16-7-4-9-18-16/h1-7,9,11,18H,8,10,12-13H2 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 1.10E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Università di Bologna
Curated by ChEMBL
| Assay Description
Inhibitory concentration required against acetylcholinesterase activity |
J Med Chem 44: 4011-4 (2001)
BindingDB Entry DOI: 10.7270/Q298869Q |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Homo sapiens (Human)) | BDBM50106447
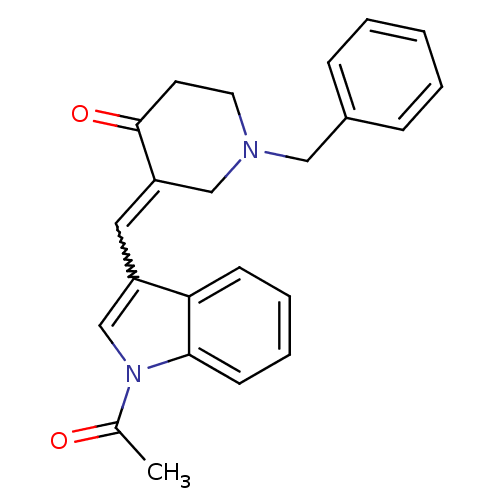
(3-(1-Acetyl-1H-indol-3-ylmethylene)-1-benzyl-piper...)Show SMILES CC(=O)n1cc(C=C2CN(Cc3ccccc3)CCC2=O)c2ccccc12 |w:6.5| Show InChI InChI=1S/C23H22N2O2/c1-17(26)25-16-19(21-9-5-6-10-22(21)25)13-20-15-24(12-11-23(20)27)14-18-7-3-2-4-8-18/h2-10,13,16H,11-12,14-15H2,1H3 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 1.10E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Università di Bologna
Curated by ChEMBL
| Assay Description
Inhibitory concentration required against acetylcholinesterase activity |
J Med Chem 44: 4011-4 (2001)
BindingDB Entry DOI: 10.7270/Q298869Q |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Homo sapiens (Human)) | BDBM50106459
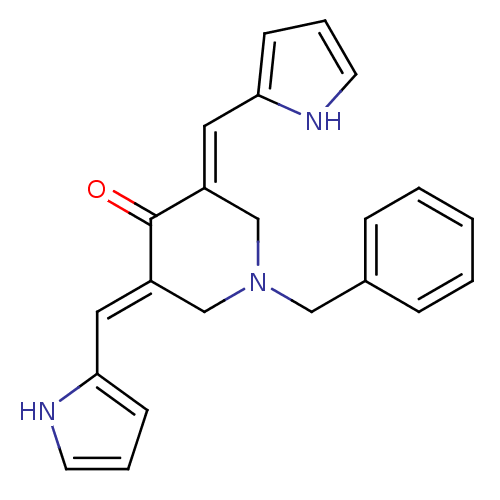
(1-Benzyl-3,5-bis-(1H-pyrrol-2-ylmethylene)-piperid...)Show SMILES O=C1\C(CN(Cc2ccccc2)C\C1=C/c1ccc[nH]1)=C\c1ccc[nH]1 Show InChI InChI=1S/C22H21N3O/c26-22-18(12-20-8-4-10-23-20)15-25(14-17-6-2-1-3-7-17)16-19(22)13-21-9-5-11-24-21/h1-13,23-24H,14-16H2/b18-12+,19-13+ | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 1.80E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Università di Bologna
Curated by ChEMBL
| Assay Description
Inhibitory concentration required against acetylcholinesterase activity |
J Med Chem 44: 4011-4 (2001)
BindingDB Entry DOI: 10.7270/Q298869Q |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Homo sapiens (Human)) | BDBM50106460
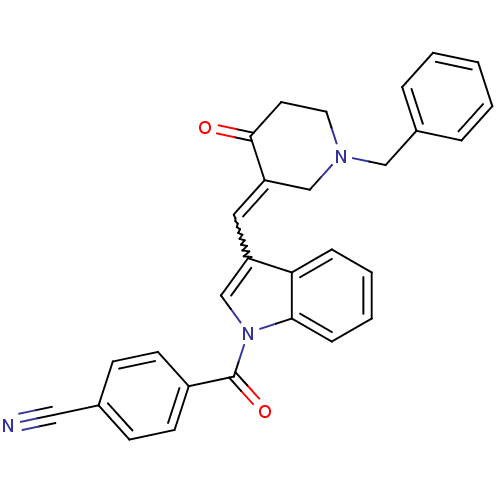
(4-[3-(1-Benzyl-4-oxo-piperidin-3-ylidenemethyl)-in...)Show SMILES O=C(c1ccc(cc1)C#N)n1cc(C=C2CN(Cc3ccccc3)CCC2=O)c2ccccc12 |w:13.13| Show InChI InChI=1S/C29H23N3O2/c30-17-21-10-12-23(13-11-21)29(34)32-20-24(26-8-4-5-9-27(26)32)16-25-19-31(15-14-28(25)33)18-22-6-2-1-3-7-22/h1-13,16,20H,14-15,18-19H2 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 2.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Università di Bologna
Curated by ChEMBL
| Assay Description
Inhibitory concentration required against acetylcholinesterase activity |
J Med Chem 44: 4011-4 (2001)
BindingDB Entry DOI: 10.7270/Q298869Q |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Homo sapiens (Human)) | BDBM50106450

(3-(1-Benzoyl-1H-pyrrol-3-ylmethylene)-1-benzyl-pip...)Show SMILES O=C1CCN(Cc2ccccc2)CC1=Cc1c[nH]c2ccc(OCc3ccccc3)cc12 |w:14.16| Show InChI InChI=1S/C28H26N2O2/c31-28-13-14-30(18-21-7-3-1-4-8-21)19-24(28)15-23-17-29-27-12-11-25(16-26(23)27)32-20-22-9-5-2-6-10-22/h1-12,15-17,29H,13-14,18-20H2 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 2.50E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Università di Bologna
Curated by ChEMBL
| Assay Description
Inhibitory concentration required against acetylcholinesterase activity |
J Med Chem 44: 4011-4 (2001)
BindingDB Entry DOI: 10.7270/Q298869Q |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Homo sapiens (Human)) | BDBM50106448
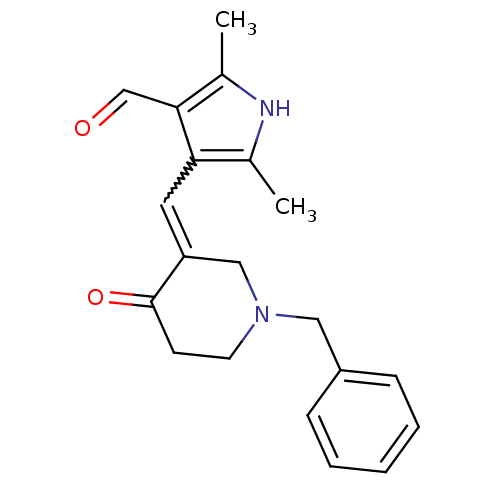
(4-(1-Benzyl-4-oxo-piperidin-3-ylidenemethyl)-2,5-d...)Show SMILES Cc1[nH]c(C)c(C=C2CN(Cc3ccccc3)CCC2=O)c1C=O |w:6.5| Show InChI InChI=1S/C20H22N2O2/c1-14-18(19(13-23)15(2)21-14)10-17-12-22(9-8-20(17)24)11-16-6-4-3-5-7-16/h3-7,10,13,21H,8-9,11-12H2,1-2H3 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| PubMed
| n/a | n/a | 3.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Università di Bologna
Curated by ChEMBL
| Assay Description
Inhibitory concentration required against acetylcholinesterase activity |
J Med Chem 44: 4011-4 (2001)
BindingDB Entry DOI: 10.7270/Q298869Q |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Homo sapiens (Human)) | BDBM50106453
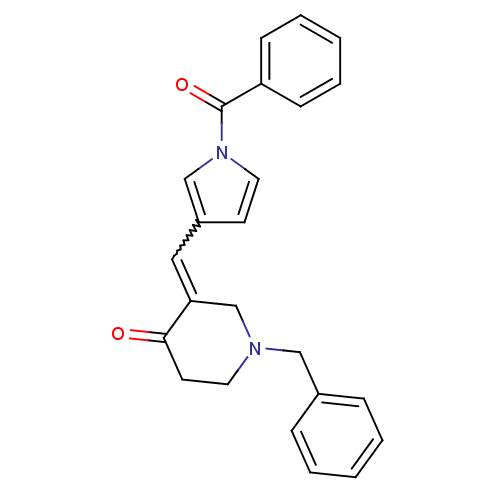
(3-(1-Benzoyl-1H-pyrrol-3-ylmethylene)-1-benzyl-pip...)Show SMILES O=C(c1ccccc1)n1ccc(C=C2CN(Cc3ccccc3)CCC2=O)c1 |w:12.12| Show InChI InChI=1S/C24H22N2O2/c27-23-12-13-25(16-19-7-3-1-4-8-19)18-22(23)15-20-11-14-26(17-20)24(28)21-9-5-2-6-10-21/h1-11,14-15,17H,12-13,16,18H2 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 3.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Università di Bologna
Curated by ChEMBL
| Assay Description
Inhibitory concentration required against acetylcholinesterase activity |
J Med Chem 44: 4011-4 (2001)
BindingDB Entry DOI: 10.7270/Q298869Q |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Homo sapiens (Human)) | BDBM50106456

(3-(1-Benzoyl-1H-pyrrol-2-ylmethylene)-1-benzyl-pip...)Show SMILES O=C(c1ccccc1)n1cccc1C=C1CN(Cc2ccccc2)CCC1=O |w:13.14| Show InChI InChI=1S/C24H22N2O2/c27-23-13-15-25(17-19-8-3-1-4-9-19)18-21(23)16-22-12-7-14-26(22)24(28)20-10-5-2-6-11-20/h1-12,14,16H,13,15,17-18H2 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| PubMed
| n/a | n/a | 3.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Università di Bologna
Curated by ChEMBL
| Assay Description
Inhibitory concentration required against acetylcholinesterase activity |
J Med Chem 44: 4011-4 (2001)
BindingDB Entry DOI: 10.7270/Q298869Q |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Homo sapiens (Human)) | BDBM50106463
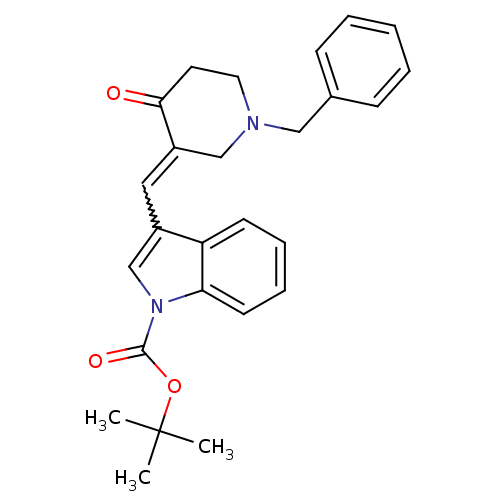
(3-(1-Benzyl-4-oxo-piperidin-3-ylidenemethyl)-indol...)Show SMILES CC(C)(C)OC(=O)n1cc(C=C2CN(Cc3ccccc3)CCC2=O)c2ccccc12 |w:10.9| Show InChI InChI=1S/C26H28N2O3/c1-26(2,3)31-25(30)28-18-20(22-11-7-8-12-23(22)28)15-21-17-27(14-13-24(21)29)16-19-9-5-4-6-10-19/h4-12,15,18H,13-14,16-17H2,1-3H3 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 3.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Università di Bologna
Curated by ChEMBL
| Assay Description
Inhibitory concentration required against acetylcholinesterase activity |
J Med Chem 44: 4011-4 (2001)
BindingDB Entry DOI: 10.7270/Q298869Q |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Homo sapiens (Human)) | BDBM50106451
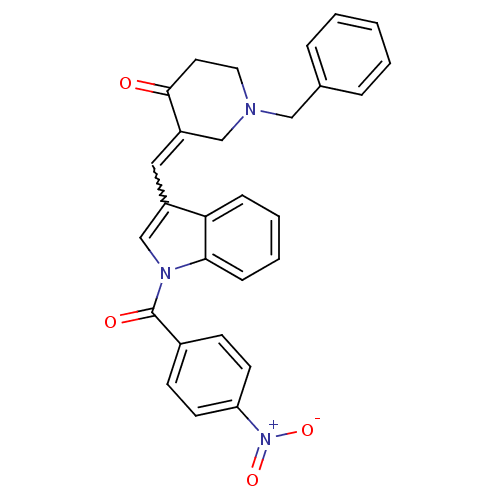
(1-Benzyl-3-[1-(4-nitro-benzoyl)-1H-indol-3-ylmethy...)Show SMILES [O-][N+](=O)c1ccc(cc1)C(=O)n1cc(C=C2CN(Cc3ccccc3)CCC2=O)c2ccccc12 |w:14.14| Show InChI InChI=1S/C28H23N3O4/c32-27-14-15-29(17-20-6-2-1-3-7-20)18-23(27)16-22-19-30(26-9-5-4-8-25(22)26)28(33)21-10-12-24(13-11-21)31(34)35/h1-13,16,19H,14-15,17-18H2 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 5.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Università di Bologna
Curated by ChEMBL
| Assay Description
Inhibitory concentration required against acetylcholinesterase activity |
J Med Chem 44: 4011-4 (2001)
BindingDB Entry DOI: 10.7270/Q298869Q |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Homo sapiens (Human)) | BDBM50106449
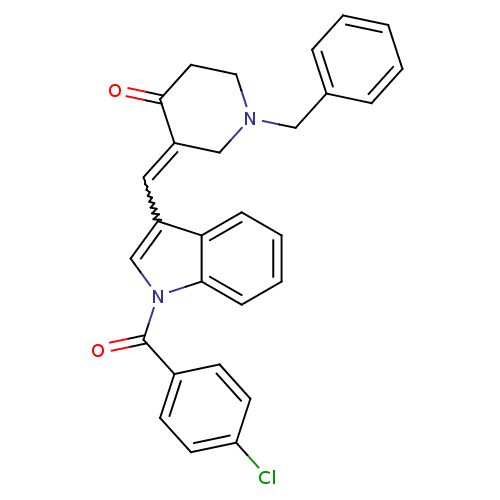
(1-Benzyl-3-[1-(4-chloro-benzoyl)-1H-indol-3-ylmeth...)Show SMILES Clc1ccc(cc1)C(=O)n1cc(C=C2CN(Cc3ccccc3)CCC2=O)c2ccccc12 |w:12.12| Show InChI InChI=1S/C28H23ClN2O2/c29-24-12-10-21(11-13-24)28(33)31-19-22(25-8-4-5-9-26(25)31)16-23-18-30(15-14-27(23)32)17-20-6-2-1-3-7-20/h1-13,16,19H,14-15,17-18H2 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 5.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Università di Bologna
Curated by ChEMBL
| Assay Description
Inhibitory concentration required against acetylcholinesterase activity |
J Med Chem 44: 4011-4 (2001)
BindingDB Entry DOI: 10.7270/Q298869Q |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Homo sapiens (Human)) | BDBM50106458

(1-Benzyl-3-[1-(4-fluoro-benzoyl)-1H-indol-3-ylmeth...)Show SMILES Fc1ccc(cc1)C(=O)n1cc(C=C2CN(Cc3ccccc3)CCC2=O)c2ccccc12 |w:12.12| Show InChI InChI=1S/C28H23FN2O2/c29-24-12-10-21(11-13-24)28(33)31-19-22(25-8-4-5-9-26(25)31)16-23-18-30(15-14-27(23)32)17-20-6-2-1-3-7-20/h1-13,16,19H,14-15,17-18H2 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 6.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Università di Bologna
Curated by ChEMBL
| Assay Description
Inhibitory concentration required against acetylcholinesterase activity |
J Med Chem 44: 4011-4 (2001)
BindingDB Entry DOI: 10.7270/Q298869Q |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Homo sapiens (Human)) | BDBM50106466
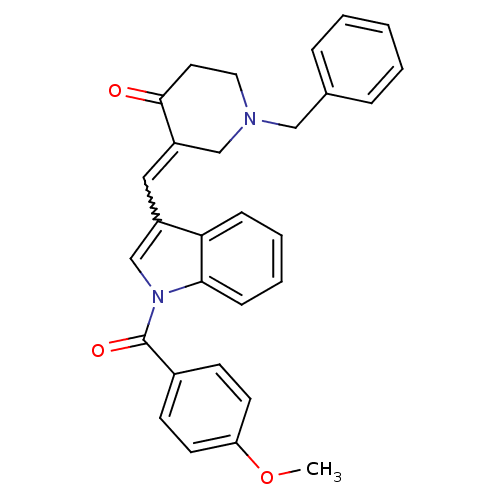
(1-Benzyl-3-[1-(4-methoxy-benzoyl)-1H-indol-3-ylmet...)Show SMILES COc1ccc(cc1)C(=O)n1cc(C=C2CN(Cc3ccccc3)CCC2=O)c2ccccc12 |w:13.13| Show InChI InChI=1S/C29H26N2O3/c1-34-25-13-11-22(12-14-25)29(33)31-20-23(26-9-5-6-10-27(26)31)17-24-19-30(16-15-28(24)32)18-21-7-3-2-4-8-21/h2-14,17,20H,15-16,18-19H2,1H3 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 7.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Università di Bologna
Curated by ChEMBL
| Assay Description
Inhibitory concentration required against acetylcholinesterase activity |
J Med Chem 44: 4011-4 (2001)
BindingDB Entry DOI: 10.7270/Q298869Q |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Homo sapiens (Human)) | BDBM50106452
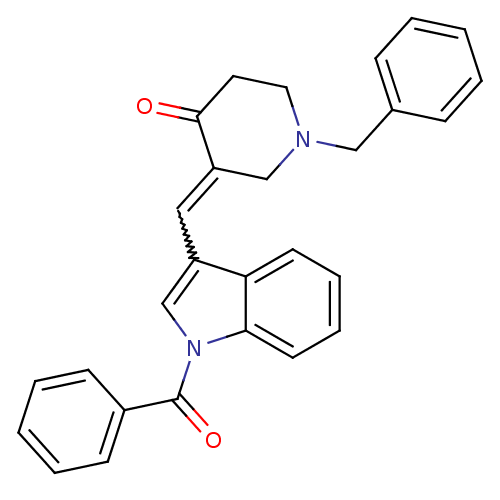
(3-(1-Benzoyl-1H-indol-3-ylmethylene)-1-benzyl-pipe...)Show SMILES O=C(c1ccccc1)n1cc(C=C2CN(Cc3ccccc3)CCC2=O)c2ccccc12 |w:11.11| Show InChI InChI=1S/C28H24N2O2/c31-27-15-16-29(18-21-9-3-1-4-10-21)19-24(27)17-23-20-30(26-14-8-7-13-25(23)26)28(32)22-11-5-2-6-12-22/h1-14,17,20H,15-16,18-19H2 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 7.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Università di Bologna
Curated by ChEMBL
| Assay Description
Inhibitory concentration required against acetylcholinesterase activity |
J Med Chem 44: 4011-4 (2001)
BindingDB Entry DOI: 10.7270/Q298869Q |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Homo sapiens (Human)) | BDBM50106464
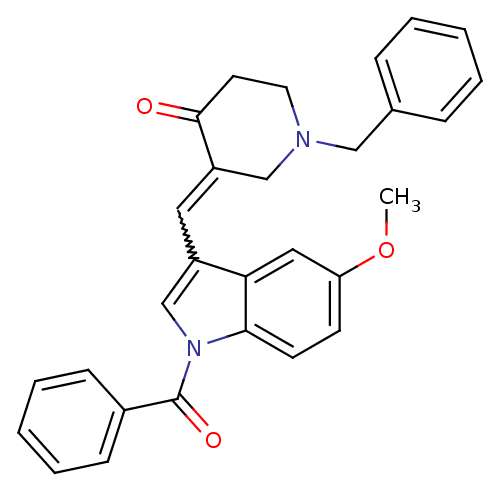
(3-(1-Benzoyl-5-methoxy-1H-indol-3-ylmethylene)-1-b...)Show SMILES COc1ccc2n(cc(C=C3CN(Cc4ccccc4)CCC3=O)c2c1)C(=O)c1ccccc1 |w:9.8| Show InChI InChI=1S/C29H26N2O3/c1-34-25-12-13-27-26(17-25)23(20-31(27)29(33)22-10-6-3-7-11-22)16-24-19-30(15-14-28(24)32)18-21-8-4-2-5-9-21/h2-13,16-17,20H,14-15,18-19H2,1H3 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 8.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Università di Bologna
Curated by ChEMBL
| Assay Description
Inhibitory concentration required against acetylcholinesterase activity |
J Med Chem 44: 4011-4 (2001)
BindingDB Entry DOI: 10.7270/Q298869Q |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Homo sapiens (Human)) | BDBM50106461

(1-Benzyl-3-(1-benzyl-1H-indol-3-ylmethylene)-piper...)Show SMILES O=C1CCN(Cc2ccccc2)CC1=Cc1cn(Cc2ccccc2)c2ccccc12 |w:14.16| Show InChI InChI=1S/C28H26N2O/c31-28-15-16-29(18-22-9-3-1-4-10-22)20-25(28)17-24-21-30(19-23-11-5-2-6-12-23)27-14-8-7-13-26(24)27/h1-14,17,21H,15-16,18-20H2 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Università di Bologna
Curated by ChEMBL
| Assay Description
Inhibitory concentration required against acetylcholinesterase activity |
J Med Chem 44: 4011-4 (2001)
BindingDB Entry DOI: 10.7270/Q298869Q |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data