

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Genome polyprotein (Human rhinovirus B) | BDBM50111455 ((1S,5S)-4-((S)-3-(3,4-Difluoro-phenyl)-2-{3-[(5-me...) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | n/a | n/a | 85 | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development-La Jolla/Agouron Pharmaceuticals Curated by ChEMBL | Assay Description antirhinoviral activity against HRV Protease 3CP (serotype 10) | J Med Chem 45: 1607-23 (2002) BindingDB Entry DOI: 10.7270/Q27M078G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Genome polyprotein (Human rhinovirus B) | BDBM50111455 ((1S,5S)-4-((S)-3-(3,4-Difluoro-phenyl)-2-{3-[(5-me...) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | n/a | n/a | 132 | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development-La Jolla/Agouron Pharmaceuticals Curated by ChEMBL | Assay Description antirhinoviral activity against HRV Protease 3CP (serotype 1A) | J Med Chem 45: 1607-23 (2002) BindingDB Entry DOI: 10.7270/Q27M078G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Genome polyprotein (Human rhinovirus B) | BDBM50110351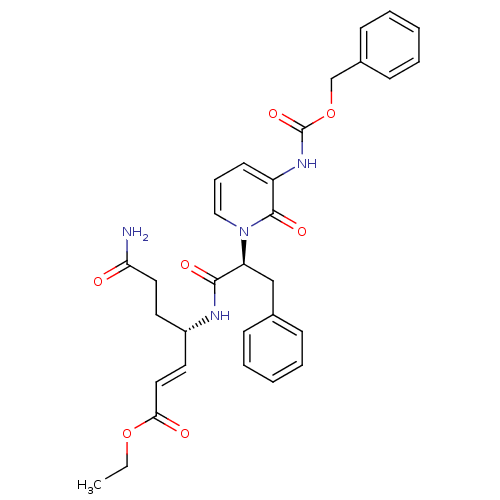 ((E)-(S)-4-[(S)-2-(3-Benzyloxycarbonylamino-2-oxo-2...) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | n/a | n/a | 33 | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development-La Jolla/Agouron Pharmaceuticals Curated by ChEMBL | Assay Description antirhinoviral activity against Human Rhinovirus Protease 3C (serotype 14) | J Med Chem 45: 1607-23 (2002) BindingDB Entry DOI: 10.7270/Q27M078G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Genome polyprotein (Human rhinovirus B) | BDBM50110353 ((E)-(S)-4-[2-(3-Benzyloxycarbonylamino-2-oxo-2H-py...) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development-La Jolla/Agouron Pharmaceuticals Curated by ChEMBL | Assay Description Inhibition of HRV Protease 3CP (serotype 14). | J Med Chem 45: 1607-23 (2002) BindingDB Entry DOI: 10.7270/Q27M078G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Genome polyprotein (Human rhinovirus B) | BDBM50111459 (6-Carbamoyl-4-(2-{2-oxo-3-[(tetrahydro-furan-2-car...) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | n/a | n/a | 1.78E+3 | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development-La Jolla/Agouron Pharmaceuticals Curated by ChEMBL | Assay Description Inhibition of HRV Protease 3CP (serotype 14). | J Med Chem 45: 1607-23 (2002) BindingDB Entry DOI: 10.7270/Q27M078G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Genome polyprotein (Human rhinovirus B) | BDBM50065606 ((E)-(S)-4-[(S)-2-((S)-2-Benzyloxycarbonylamino-4-m...) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | n/a | n/a | 540 | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development-La Jolla/Agouron Pharmaceuticals Curated by ChEMBL | Assay Description antirhinoviral activity against Human Rhinovirus Protease 3C (serotype 14) | J Med Chem 45: 1607-23 (2002) BindingDB Entry DOI: 10.7270/Q27M078G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Genome polyprotein (Human rhinovirus B) | BDBM50111465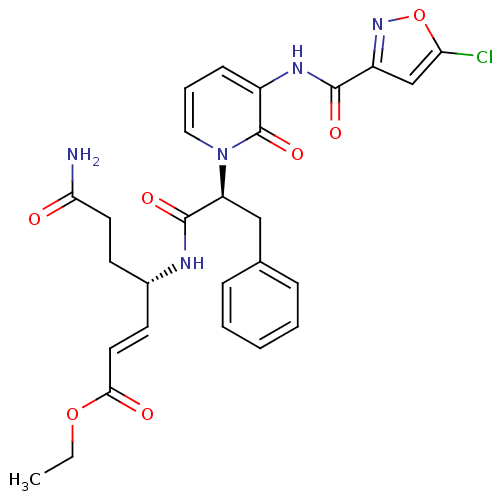 (6-Carbamoyl-4-(2-{3-[(5-chloro-isoxazole-3-carbony...) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | n/a | n/a | 24 | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development-La Jolla/Agouron Pharmaceuticals Curated by ChEMBL | Assay Description Inhibition of HRV Protease 3CP (serotype 14). | J Med Chem 45: 1607-23 (2002) BindingDB Entry DOI: 10.7270/Q27M078G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Genome polyprotein (Human rhinovirus B) | BDBM50111464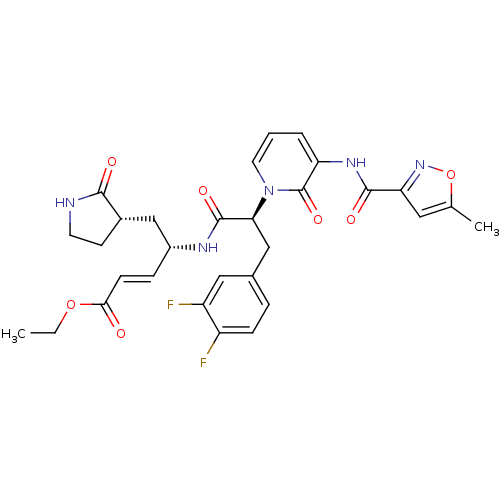 ((1S,5S)-4-((S)-3-(3,4-Difluoro-phenyl)-2-{3-[(5-me...) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | n/a | n/a | 1 | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development-La Jolla/Agouron Pharmaceuticals Curated by ChEMBL | Assay Description Inhibition of HRV Protease 3CP (serotype 14). | J Med Chem 45: 1607-23 (2002) BindingDB Entry DOI: 10.7270/Q27M078G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Genome polyprotein (Human rhinovirus B) | BDBM50111462 (6-Carbamoyl-4-{2-[3-(cyclopentanecarbonyl-amino)-2...) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | n/a | n/a | 34 | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development-La Jolla/Agouron Pharmaceuticals Curated by ChEMBL | Assay Description Inhibition of HRV Protease 3CP (serotype 14). | J Med Chem 45: 1607-23 (2002) BindingDB Entry DOI: 10.7270/Q27M078G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Genome polyprotein (Human rhinovirus B) | BDBM50111456 (4-[2-(3-Benzyloxycarbonylamino-2-oxo-2H-pyridin-1-...) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | n/a | n/a | 607 | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development-La Jolla/Agouron Pharmaceuticals Curated by ChEMBL | Assay Description antirhinoviral activity against HRV Protease 3CP (serotype 1A) | J Med Chem 45: 1607-23 (2002) BindingDB Entry DOI: 10.7270/Q27M078G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Genome polyprotein (Human rhinovirus B) | BDBM50111455 ((1S,5S)-4-((S)-3-(3,4-Difluoro-phenyl)-2-{3-[(5-me...) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | n/a | n/a | 3 | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development-La Jolla/Agouron Pharmaceuticals Curated by ChEMBL | Assay Description antirhinoviral activity against HRV Protease 3CP (serotype Hanks) | J Med Chem 45: 1607-23 (2002) BindingDB Entry DOI: 10.7270/Q27M078G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Genome polyprotein (Human rhinovirus B) | BDBM50111469 (3-(3,5-dimethyl-4-(3-(3-methylisoxazol-5-yl)propox...) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE MMDB PC cid PC sid PDB UniChem Patents | PubMed | n/a | n/a | n/a | n/a | 58 | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development-La Jolla/Agouron Pharmaceuticals Curated by ChEMBL | Assay Description Inhibition of HRV Protease 3CP (serotype 14). | J Med Chem 45: 1607-23 (2002) BindingDB Entry DOI: 10.7270/Q27M078G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Genome polyprotein (Human rhinovirus B) | BDBM50110351 ((E)-(S)-4-[(S)-2-(3-Benzyloxycarbonylamino-2-oxo-2...) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | n/a | n/a | 1.25E+3 | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development-La Jolla/Agouron Pharmaceuticals Curated by ChEMBL | Assay Description antirhinoviral activity against Human Rhinovirus Protease 3C (serotype 1A) | J Med Chem 45: 1607-23 (2002) BindingDB Entry DOI: 10.7270/Q27M078G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Genome polyprotein (Human rhinovirus B) | BDBM50111455 ((1S,5S)-4-((S)-3-(3,4-Difluoro-phenyl)-2-{3-[(5-me...) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | n/a | n/a | 44 | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development-La Jolla/Agouron Pharmaceuticals Curated by ChEMBL | Assay Description antirhinoviral activity against HRV Protease 3CP (serotype 13) | J Med Chem 45: 1607-23 (2002) BindingDB Entry DOI: 10.7270/Q27M078G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Genome polyprotein (Human rhinovirus B) | BDBM50111455 ((1S,5S)-4-((S)-3-(3,4-Difluoro-phenyl)-2-{3-[(5-me...) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | n/a | n/a | 10 | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development-La Jolla/Agouron Pharmaceuticals Curated by ChEMBL | Assay Description antirhinoviral activity against HRV Protease 3CP (serotype 23) | J Med Chem 45: 1607-23 (2002) BindingDB Entry DOI: 10.7270/Q27M078G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Genome polyprotein (Human rhinovirus B) | BDBM50111455 ((1S,5S)-4-((S)-3-(3,4-Difluoro-phenyl)-2-{3-[(5-me...) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | n/a | n/a | 109 | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development-La Jolla/Agouron Pharmaceuticals Curated by ChEMBL | Assay Description antirhinoviral activity against HRV Protease 3CP (serotype 9) | J Med Chem 45: 1607-23 (2002) BindingDB Entry DOI: 10.7270/Q27M078G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Genome polyprotein (Human rhinovirus B) | BDBM50111455 ((1S,5S)-4-((S)-3-(3,4-Difluoro-phenyl)-2-{3-[(5-me...) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | n/a | n/a | 36 | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development-La Jolla/Agouron Pharmaceuticals Curated by ChEMBL | Assay Description antirhinoviral activity against HRV Protease 3CP (serotype 16) | J Med Chem 45: 1607-23 (2002) BindingDB Entry DOI: 10.7270/Q27M078G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Genome polyprotein (Human rhinovirus B) | BDBM50111461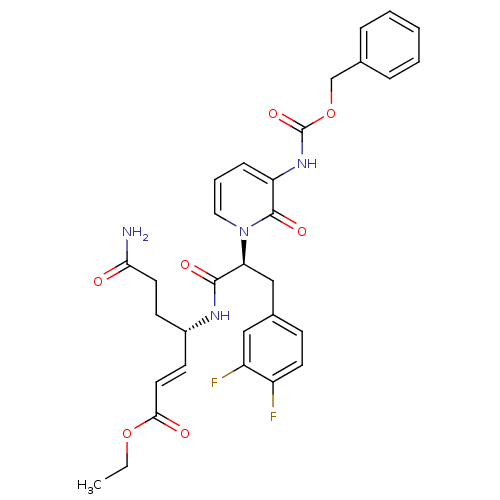 (4-[2-(3-Benzyloxycarbonylamino-2-oxo-2H-pyridin-1-...) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | n/a | n/a | 3 | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development-La Jolla/Agouron Pharmaceuticals Curated by ChEMBL | Assay Description Inhibition of HRV Protease 3CP (serotype 14). | J Med Chem 45: 1607-23 (2002) BindingDB Entry DOI: 10.7270/Q27M078G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Genome polyprotein (Human rhinovirus B) | BDBM50111455 ((1S,5S)-4-((S)-3-(3,4-Difluoro-phenyl)-2-{3-[(5-me...) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | n/a | n/a | 12 | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development-La Jolla/Agouron Pharmaceuticals Curated by ChEMBL | Assay Description antirhinoviral activity against HRV Protease 3CP (serotype 78) | J Med Chem 45: 1607-23 (2002) BindingDB Entry DOI: 10.7270/Q27M078G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Genome polyprotein (Human rhinovirus B) | BDBM50087765 ((METHYLPYRIDAZINE PIPERIDINE ETHYLOXYPHENYL)ETHYLA...) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE MMDB PC cid PC sid PDB UniChem Patents | PubMed | n/a | n/a | n/a | n/a | 440 | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development-La Jolla/Agouron Pharmaceuticals Curated by ChEMBL | Assay Description Inhibition of HRV Protease 3CP (serotype 14). | J Med Chem 45: 1607-23 (2002) BindingDB Entry DOI: 10.7270/Q27M078G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Genome polyprotein (Human rhinovirus B) | BDBM50111456 (4-[2-(3-Benzyloxycarbonylamino-2-oxo-2H-pyridin-1-...) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | n/a | n/a | 14 | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development-La Jolla/Agouron Pharmaceuticals Curated by ChEMBL | Assay Description Inhibition of HRV Protease 3CP (serotype 14). | J Med Chem 45: 1607-23 (2002) BindingDB Entry DOI: 10.7270/Q27M078G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Genome polyprotein (Human rhinovirus B) | BDBM50111456 (4-[2-(3-Benzyloxycarbonylamino-2-oxo-2H-pyridin-1-...) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | n/a | n/a | 111 | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development-La Jolla/Agouron Pharmaceuticals Curated by ChEMBL | Assay Description antirhinoviral activity against HRV Protease 3CP (serotype 10) | J Med Chem 45: 1607-23 (2002) BindingDB Entry DOI: 10.7270/Q27M078G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Genome polyprotein (Human rhinovirus B) | BDBM50111460 (4-[2-(3-Acetylamino-2-oxo-2H-pyridin-1-yl)-3-pheny...) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | n/a | n/a | 888 | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development-La Jolla/Agouron Pharmaceuticals Curated by ChEMBL | Assay Description Inhibition of HRV Protease 3CP (serotype 14). | J Med Chem 45: 1607-23 (2002) BindingDB Entry DOI: 10.7270/Q27M078G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Genome polyprotein (Human rhinovirus B) | BDBM50111464 ((1S,5S)-4-((S)-3-(3,4-Difluoro-phenyl)-2-{3-[(5-me...) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | n/a | n/a | 5 | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development-La Jolla/Agouron Pharmaceuticals Curated by ChEMBL | Assay Description antirhinoviral activity against HRV Protease 3CP (serotype 10) | J Med Chem 45: 1607-23 (2002) BindingDB Entry DOI: 10.7270/Q27M078G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Genome polyprotein (Human rhinovirus B) | BDBM50111455 ((1S,5S)-4-((S)-3-(3,4-Difluoro-phenyl)-2-{3-[(5-me...) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | n/a | n/a | 48 | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development-La Jolla/Agouron Pharmaceuticals Curated by ChEMBL | Assay Description antirhinoviral activity against HRV Protease 3CP (serotype 2) | J Med Chem 45: 1607-23 (2002) BindingDB Entry DOI: 10.7270/Q27M078G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Genome polyprotein (Human rhinovirus B) | BDBM50111455 ((1S,5S)-4-((S)-3-(3,4-Difluoro-phenyl)-2-{3-[(5-me...) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | n/a | n/a | 3 | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development-La Jolla/Agouron Pharmaceuticals Curated by ChEMBL | Assay Description antirhinoviral activity against HRV Protease 3CP (serotype 11) | J Med Chem 45: 1607-23 (2002) BindingDB Entry DOI: 10.7270/Q27M078G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Genome polyprotein (Human rhinovirus B) | BDBM50111461 (4-[2-(3-Benzyloxycarbonylamino-2-oxo-2H-pyridin-1-...) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | n/a | n/a | 32 | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development-La Jolla/Agouron Pharmaceuticals Curated by ChEMBL | Assay Description antirhinoviral activity against HRV Protease 3CP (serotype 10) | J Med Chem 45: 1607-23 (2002) BindingDB Entry DOI: 10.7270/Q27M078G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Genome polyprotein (Human rhinovirus B) | BDBM50111467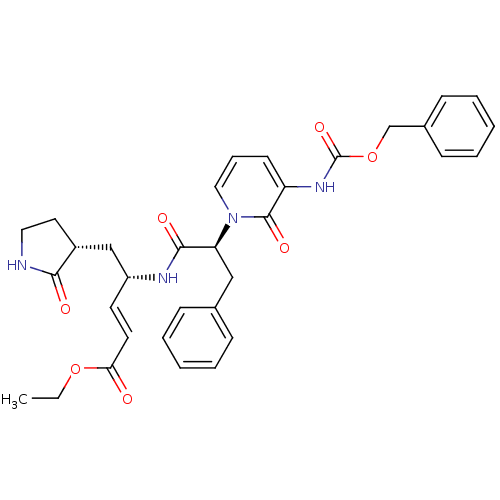 (4-[2-(3-Benzyloxycarbonylamino-2-oxo-2H-pyridin-1-...) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | n/a | n/a | 469 | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development-La Jolla/Agouron Pharmaceuticals Curated by ChEMBL | Assay Description Inhibition of HRV Protease 3CP (serotype 14). | J Med Chem 45: 1607-23 (2002) BindingDB Entry DOI: 10.7270/Q27M078G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Genome polyprotein (Human rhinovirus B) | BDBM50111455 ((1S,5S)-4-((S)-3-(3,4-Difluoro-phenyl)-2-{3-[(5-me...) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | n/a | n/a | 33 | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development-La Jolla/Agouron Pharmaceuticals Curated by ChEMBL | Assay Description antirhinoviral activity against HRV Protease 3CP (serotype 3) | J Med Chem 45: 1607-23 (2002) BindingDB Entry DOI: 10.7270/Q27M078G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Genome polyprotein (Human rhinovirus B) | BDBM50111463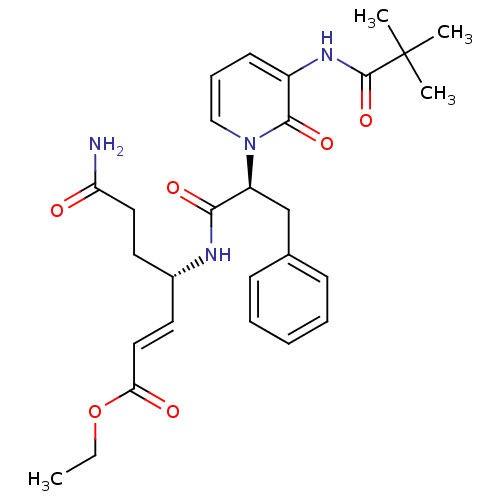 (6-Carbamoyl-4-{2-[3-(2,2-dimethyl-propionylamino)-...) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | n/a | n/a | 518 | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development-La Jolla/Agouron Pharmaceuticals Curated by ChEMBL | Assay Description Inhibition of HRV Protease 3CP (serotype 14). | J Med Chem 45: 1607-23 (2002) BindingDB Entry DOI: 10.7270/Q27M078G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Genome polyprotein (Human rhinovirus B) | BDBM50111458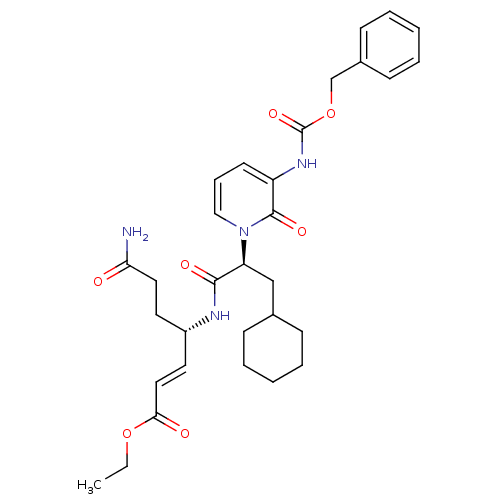 (4-[2-(3-Benzyloxycarbonylamino-2-oxo-2H-pyridin-1-...) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | n/a | n/a | 178 | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development-La Jolla/Agouron Pharmaceuticals Curated by ChEMBL | Assay Description Inhibition of HRV Protease 3CP (serotype 14). | J Med Chem 45: 1607-23 (2002) BindingDB Entry DOI: 10.7270/Q27M078G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Genome polyprotein (Human rhinovirus B) | BDBM50111466 (4-[2-(3-Benzyloxycarbonylamino-2-oxo-2H-pyridin-1-...) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | n/a | n/a | 3 | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development-La Jolla/Agouron Pharmaceuticals Curated by ChEMBL | Assay Description antirhinoviral activity against HRV Protease 3CP (serotype 14) | J Med Chem 45: 1607-23 (2002) BindingDB Entry DOI: 10.7270/Q27M078G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Genome polyprotein (Human rhinovirus B) | BDBM50111454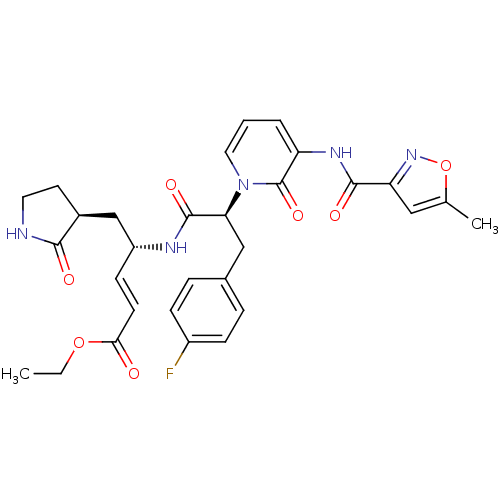 (4-(3-(4-Fluoro-phenyl)-2-{3-[(5-methyl-isoxazole-3...) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | n/a | n/a | 2 | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development-La Jolla/Agouron Pharmaceuticals Curated by ChEMBL | Assay Description Inhibition of HRV Protease 3CP (serotype 14). | J Med Chem 45: 1607-23 (2002) BindingDB Entry DOI: 10.7270/Q27M078G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Genome polyprotein (Human rhinovirus B) | BDBM50111454 (4-(3-(4-Fluoro-phenyl)-2-{3-[(5-methyl-isoxazole-3...) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | n/a | n/a | 15 | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development-La Jolla/Agouron Pharmaceuticals Curated by ChEMBL | Assay Description antirhinoviral activity against HRV Protease 3CP (serotype 1A) | J Med Chem 45: 1607-23 (2002) BindingDB Entry DOI: 10.7270/Q27M078G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Genome polyprotein (Human rhinovirus B) | BDBM50111457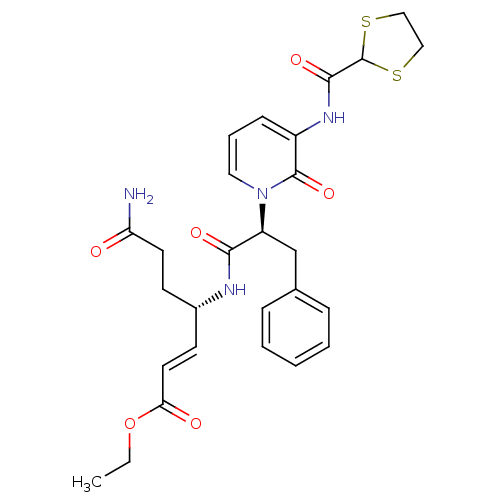 (6-Carbamoyl-4-(2-{3-[([1,3]dithiolane-2-carbonyl)-...) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | n/a | n/a | 1.18E+3 | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development-La Jolla/Agouron Pharmaceuticals Curated by ChEMBL | Assay Description antirhinoviral activity against HRV Protease 3CP (serotype 1A) | J Med Chem 45: 1607-23 (2002) BindingDB Entry DOI: 10.7270/Q27M078G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Genome polyprotein (Human rhinovirus B) | BDBM50111454 (4-(3-(4-Fluoro-phenyl)-2-{3-[(5-methyl-isoxazole-3...) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | n/a | n/a | 4 | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development-La Jolla/Agouron Pharmaceuticals Curated by ChEMBL | Assay Description antirhinoviral activity against HRV Protease 3CP (serotype 10) | J Med Chem 45: 1607-23 (2002) BindingDB Entry DOI: 10.7270/Q27M078G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Genome polyprotein (Human rhinovirus B) | BDBM50111464 ((1S,5S)-4-((S)-3-(3,4-Difluoro-phenyl)-2-{3-[(5-me...) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | n/a | n/a | 6 | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development-La Jolla/Agouron Pharmaceuticals Curated by ChEMBL | Assay Description antirhinoviral activity against HRV Protease 3CP (serotype 1A) | J Med Chem 45: 1607-23 (2002) BindingDB Entry DOI: 10.7270/Q27M078G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Genome polyprotein (Human rhinovirus B) | BDBM50111461 (4-[2-(3-Benzyloxycarbonylamino-2-oxo-2H-pyridin-1-...) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | n/a | n/a | 513 | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development-La Jolla/Agouron Pharmaceuticals Curated by ChEMBL | Assay Description antirhinoviral activity against HRV Protease 3CP (serotype 1A) | J Med Chem 45: 1607-23 (2002) BindingDB Entry DOI: 10.7270/Q27M078G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Genome polyprotein (Human rhinovirus B) | BDBM50111455 ((1S,5S)-4-((S)-3-(3,4-Difluoro-phenyl)-2-{3-[(5-me...) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | n/a | n/a | 9 | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development-La Jolla/Agouron Pharmaceuticals Curated by ChEMBL | Assay Description antirhinoviral activity against HRV Protease 3CP (serotype 19) | J Med Chem 45: 1607-23 (2002) BindingDB Entry DOI: 10.7270/Q27M078G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Genome polyprotein (Human rhinovirus B) | BDBM50111468 (6-Carbamoyl-4-(2-{3-[(5-methyl-isoxazole-3-carbony...) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | n/a | n/a | 16 | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development-La Jolla/Agouron Pharmaceuticals Curated by ChEMBL | Assay Description Inhibition of HRV Protease 3CP (serotype 14). | J Med Chem 45: 1607-23 (2002) BindingDB Entry DOI: 10.7270/Q27M078G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Genome polyprotein (Human rhinovirus B) | BDBM50111455 ((1S,5S)-4-((S)-3-(3,4-Difluoro-phenyl)-2-{3-[(5-me...) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | n/a | n/a | 3 | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development-La Jolla/Agouron Pharmaceuticals Curated by ChEMBL | Assay Description antirhinoviral activity against HRV Protease 3CP (serotype 14) | J Med Chem 45: 1607-23 (2002) BindingDB Entry DOI: 10.7270/Q27M078G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Genome polyprotein (Human rhinovirus B) | BDBM50111457 (6-Carbamoyl-4-(2-{3-[([1,3]dithiolane-2-carbonyl)-...) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | n/a | n/a | 104 | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development-La Jolla/Agouron Pharmaceuticals Curated by ChEMBL | Assay Description Inhibition of HRV Protease 3CP (serotype 14). | J Med Chem 45: 1607-23 (2002) BindingDB Entry DOI: 10.7270/Q27M078G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Genome polyprotein (Human rhinovirus B) | BDBM50111455 ((1S,5S)-4-((S)-3-(3,4-Difluoro-phenyl)-2-{3-[(5-me...) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | n/a | n/a | 100 | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development-La Jolla/Agouron Pharmaceuticals Curated by ChEMBL | Assay Description antirhinoviral activity against HRV Protease 3CP (serotype 25) | J Med Chem 45: 1607-23 (2002) BindingDB Entry DOI: 10.7270/Q27M078G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Genome polyprotein (Human rhinovirus B) | BDBM50111457 (6-Carbamoyl-4-(2-{3-[([1,3]dithiolane-2-carbonyl)-...) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | n/a | n/a | 1.41E+3 | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development-La Jolla/Agouron Pharmaceuticals Curated by ChEMBL | Assay Description antirhinoviral activity against HRV Protease 3CP (serotype 10) | J Med Chem 45: 1607-23 (2002) BindingDB Entry DOI: 10.7270/Q27M078G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Genome polyprotein (Human rhinovirus B) | BDBM50111455 ((1S,5S)-4-((S)-3-(3,4-Difluoro-phenyl)-2-{3-[(5-me...) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | n/a | n/a | 44 | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development-La Jolla/Agouron Pharmaceuticals Curated by ChEMBL | Assay Description antirhinoviral activity against HRV Protease 3CP (serotype 39) | J Med Chem 45: 1607-23 (2002) BindingDB Entry DOI: 10.7270/Q27M078G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Genome polyprotein (Human rhinovirus B) | BDBM50110351 ((E)-(S)-4-[(S)-2-(3-Benzyloxycarbonylamino-2-oxo-2...) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | n/a | n/a | 372 | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development-La Jolla/Agouron Pharmaceuticals Curated by ChEMBL | Assay Description antirhinoviral activity against Human Rhinovirus Protease 3C (serotype 10) | J Med Chem 45: 1607-23 (2002) BindingDB Entry DOI: 10.7270/Q27M078G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||