

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Inosine-5'-monophosphate dehydrogenase 2 (Homo sapiens (Human)) | BDBM19264 ((4E)-6-(4-hydroxy-6-methoxy-7-methyl-3-oxo-1,3-dih...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE MMDB PC cid PC sid PDB UniChem Patents Similars | DrugBank PDB PubMed | n/a | n/a | 15 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute Curated by ChEMBL | Assay Description In vitro inhibition of Inosine-5'-monophosphate dehydrogenase 2. | Bioorg Med Chem Lett 12: 2931-4 (2002) BindingDB Entry DOI: 10.7270/Q2SB454K | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Inosine-5'-monophosphate dehydrogenase 2 (Homo sapiens (Human)) | BDBM50119113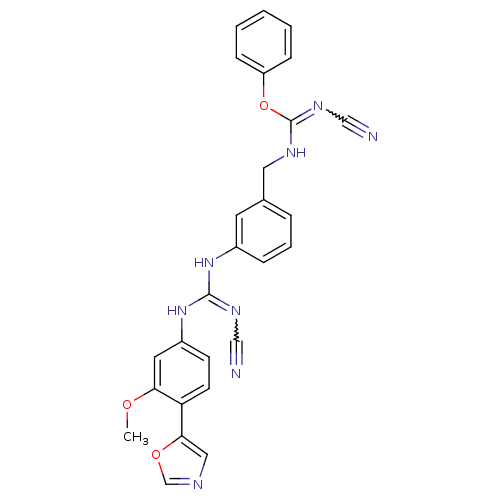 (1-{3-[N'-(3-Methoxy-4-oxazol-5-yl-phenyl)-N''-cyan...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | PubMed | n/a | n/a | 16 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute Curated by ChEMBL | Assay Description In vitro inhibition of Inosine-5'-monophosphate dehydrogenase 2. | Bioorg Med Chem Lett 12: 2931-4 (2002) BindingDB Entry DOI: 10.7270/Q2SB454K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Inosine-5'-monophosphate dehydrogenase 2 (Homo sapiens (Human)) | BDBM50119045 (1-(3-Methoxy-4-oxazol-5-yl-phenyl)-3-m-tolyl-urea ...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 19 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute Curated by ChEMBL | Assay Description In vitro inhibition of Inosine-5'-monophosphate dehydrogenase 2. | Bioorg Med Chem Lett 12: 2931-4 (2002) BindingDB Entry DOI: 10.7270/Q2SB454K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Inosine-5'-monophosphate dehydrogenase 2 (Homo sapiens (Human)) | BDBM50119128 (CHEMBL98265 | {3-[N'-(3-Methoxy-4-oxazol-5-yl-phen...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 110 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute Curated by ChEMBL | Assay Description The compound was tested for it's in vitro inhibitor potency against inosine monophosphate dehydrogenase II (IMPDH II) | Bioorg Med Chem Lett 12: 2931-4 (2002) BindingDB Entry DOI: 10.7270/Q2SB454K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Inosine-5'-monophosphate dehydrogenase 2 (Homo sapiens (Human)) | BDBM50119120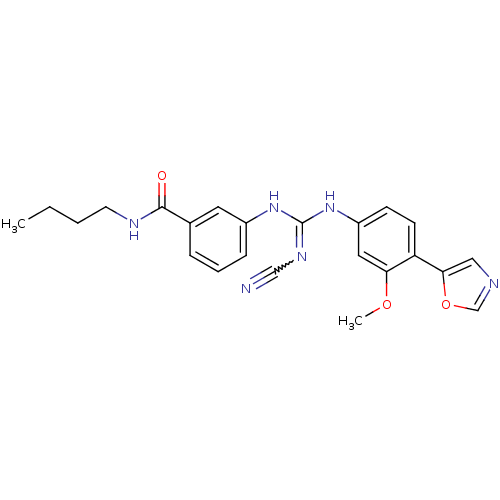 (CHEMBL101941 | N-Butyl-3-[N'-(3-methoxy-4-oxazol-5...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | PubMed | n/a | n/a | 130 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute Curated by ChEMBL | Assay Description In vitro inhibition of Inosine-5'-monophosphate dehydrogenase 2. | Bioorg Med Chem Lett 12: 2931-4 (2002) BindingDB Entry DOI: 10.7270/Q2SB454K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Inosine-5'-monophosphate dehydrogenase 2 (Homo sapiens (Human)) | BDBM50119127 (1-{3-[N'-(3-Methoxy-4-oxazol-5-yl-phenyl)-N''-cyan...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | PubMed | n/a | n/a | 180 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute Curated by ChEMBL | Assay Description In vitro inhibition of Inosine-5'-monophosphate dehydrogenase 2. | Bioorg Med Chem Lett 12: 2931-4 (2002) BindingDB Entry DOI: 10.7270/Q2SB454K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Inosine-5'-monophosphate dehydrogenase 2 (Homo sapiens (Human)) | BDBM50119119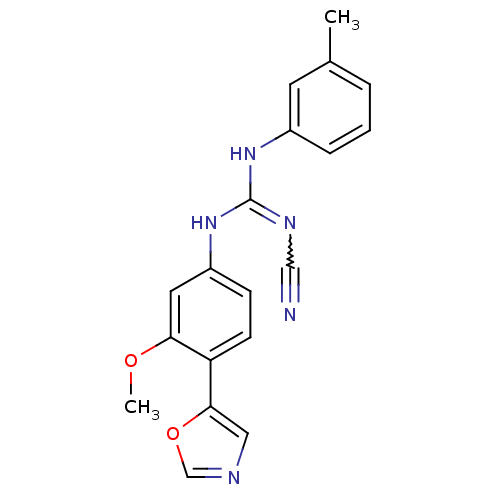 (CHEMBL98740 | N''-cyano-N-[3-methoxy-4-(1,3-oxazol...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 240 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute Curated by ChEMBL | Assay Description In vitro inhibition of Inosine-5'-monophosphate dehydrogenase 2. | Bioorg Med Chem Lett 12: 2931-4 (2002) BindingDB Entry DOI: 10.7270/Q2SB454K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Inosine-5'-monophosphate dehydrogenase 2 (Homo sapiens (Human)) | BDBM50119130 (CHEMBL60730 | N''-cyano-N-[3-methoxy-4-(1,3-oxazol...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 250 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute Curated by ChEMBL | Assay Description In vitro inhibition of Inosine-5'-monophosphate dehydrogenase 2. | Bioorg Med Chem Lett 12: 2931-4 (2002) BindingDB Entry DOI: 10.7270/Q2SB454K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Inosine-5'-monophosphate dehydrogenase 2 (Homo sapiens (Human)) | BDBM50119126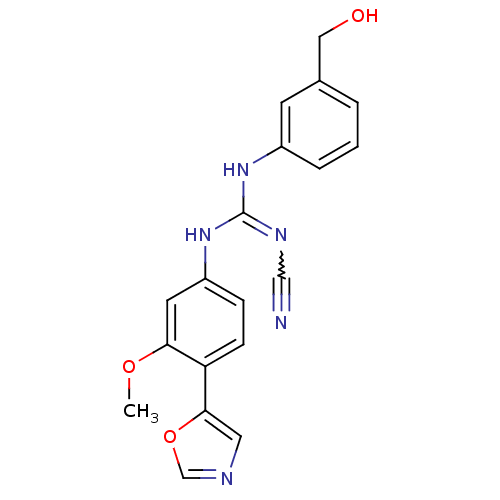 (CHEMBL99625 | N''-cyano-N-[3-(hydroxymethyl)phenyl...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 280 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute Curated by ChEMBL | Assay Description In vitro inhibition of Inosine-5'-monophosphate dehydrogenase 2. | Bioorg Med Chem Lett 12: 2931-4 (2002) BindingDB Entry DOI: 10.7270/Q2SB454K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Inosine-5'-monophosphate dehydrogenase 2 (Homo sapiens (Human)) | BDBM50119115 (CHEMBL101822 | N''-cyano-N-[3-methoxy-4-(1,3-oxazo...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 320 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute Curated by ChEMBL | Assay Description In vitro inhibition of Inosine-5'-monophosphate dehydrogenase 2. | Bioorg Med Chem Lett 12: 2931-4 (2002) BindingDB Entry DOI: 10.7270/Q2SB454K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Inosine-5'-monophosphate dehydrogenase 2 (Homo sapiens (Human)) | BDBM50119114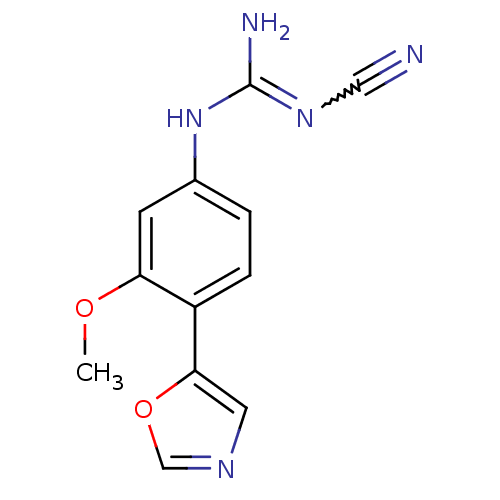 (CHEMBL98393 | N''-cyano-N-[3-methoxy-4-(1,3-oxazol...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 330 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute Curated by ChEMBL | Assay Description In vitro inhibition of Inosine-5'-monophosphate dehydrogenase 2. | Bioorg Med Chem Lett 12: 2931-4 (2002) BindingDB Entry DOI: 10.7270/Q2SB454K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Inosine-5'-monophosphate dehydrogenase 2 (Homo sapiens (Human)) | BDBM50119124 (CHEMBL101463 | {3-[N'-(3-Methoxy-4-oxazol-5-yl-phe...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 340 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute Curated by ChEMBL | Assay Description In vitro inhibition of Inosine-5'-monophosphate dehydrogenase 2. | Bioorg Med Chem Lett 12: 2931-4 (2002) BindingDB Entry DOI: 10.7270/Q2SB454K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Inosine-5'-monophosphate dehydrogenase 2 (Homo sapiens (Human)) | BDBM50119121 (CHEMBL98322 | N''-cyano-N-[3-methoxy-4-(1,3-oxazol...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 340 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute Curated by ChEMBL | Assay Description In vitro inhibition of Inosine-5'-monophosphate dehydrogenase 2. | Bioorg Med Chem Lett 12: 2931-4 (2002) BindingDB Entry DOI: 10.7270/Q2SB454K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Inosine-5'-monophosphate dehydrogenase 2 (Homo sapiens (Human)) | BDBM50119110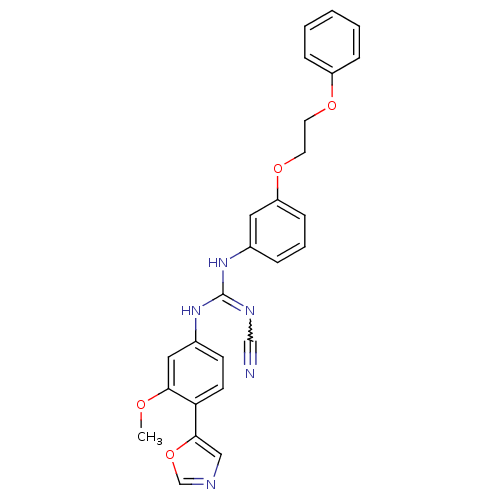 (CHEMBL99278 | N-(3-Methoxy-4-oxazol-5-yl-phenyl)-N...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 380 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute Curated by ChEMBL | Assay Description In vitro inhibition of Inosine-5'-monophosphate dehydrogenase 2. | Bioorg Med Chem Lett 12: 2931-4 (2002) BindingDB Entry DOI: 10.7270/Q2SB454K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Inosine-5'-monophosphate dehydrogenase 2 (Homo sapiens (Human)) | BDBM50119111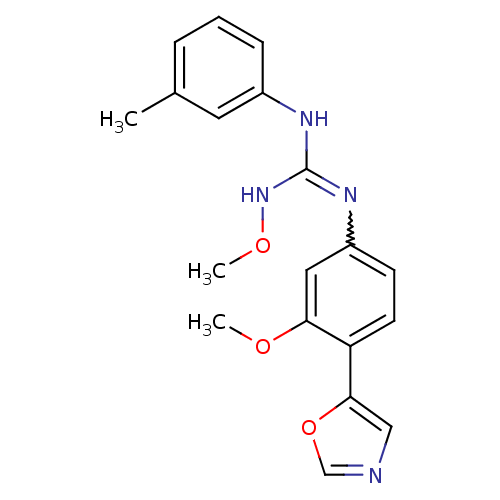 (CHEMBL98904 | N''-methoxy-N-[3-methoxy-4-(1,3-oxaz...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 660 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute Curated by ChEMBL | Assay Description In vitro inhibition of Inosine-5'-monophosphate dehydrogenase 2. | Bioorg Med Chem Lett 12: 2931-4 (2002) BindingDB Entry DOI: 10.7270/Q2SB454K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Inosine-5'-monophosphate dehydrogenase 2 (Homo sapiens (Human)) | BDBM50119129 (CHEMBL317497 | ethyl(1E)-[(3-methoxy-4-oxazole-5-y...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 740 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute Curated by ChEMBL | Assay Description In vitro inhibition of Inosine-5'-monophosphate dehydrogenase 2. | Bioorg Med Chem Lett 12: 2931-4 (2002) BindingDB Entry DOI: 10.7270/Q2SB454K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Inosine-5'-monophosphate dehydrogenase 2 (Homo sapiens (Human)) | BDBM50119123 (CHEMBL329714 | N-Benzoyl-N'-(3-methoxy-4-oxazol-5-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | PubMed | n/a | n/a | 800 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute Curated by ChEMBL | Assay Description In vitro inhibition of Inosine-5'-monophosphate dehydrogenase 2. | Bioorg Med Chem Lett 12: 2931-4 (2002) BindingDB Entry DOI: 10.7270/Q2SB454K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Inosine-5'-monophosphate dehydrogenase 2 (Homo sapiens (Human)) | BDBM50119118 (CHEMBL98225 | N-Acetyl-N'-(3-methoxy-4-oxazol-5-yl...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | >1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute Curated by ChEMBL | Assay Description In vitro inhibition of Inosine-5'-monophosphate dehydrogenase 2. | Bioorg Med Chem Lett 12: 2931-4 (2002) BindingDB Entry DOI: 10.7270/Q2SB454K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Inosine-5'-monophosphate dehydrogenase 2 (Homo sapiens (Human)) | BDBM50119125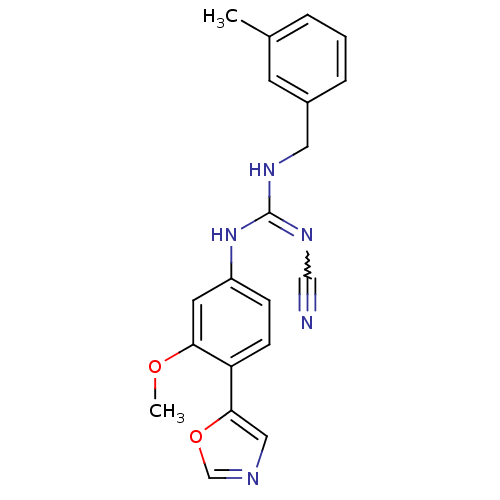 (CHEMBL98500 | N''-cyano-N-[3-methoxy-4-(1,3-oxazol...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 1.10E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute Curated by ChEMBL | Assay Description In vitro inhibition of Inosine-5'-monophosphate dehydrogenase 2. | Bioorg Med Chem Lett 12: 2931-4 (2002) BindingDB Entry DOI: 10.7270/Q2SB454K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Inosine-5'-monophosphate dehydrogenase 2 (Homo sapiens (Human)) | BDBM50119112 (CHEMBL98700 | N''-cyano-N-[3-methoxy-4-(1,3-oxazol...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 1.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute Curated by ChEMBL | Assay Description In vitro inhibition of Inosine-5'-monophosphate dehydrogenase 2. | Bioorg Med Chem Lett 12: 2931-4 (2002) BindingDB Entry DOI: 10.7270/Q2SB454K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Inosine-5'-monophosphate dehydrogenase 2 (Homo sapiens (Human)) | BDBM50119122 (CHEMBL316946 | N''-cyano-N-[3-methoxy-4-(1,3-oxazo...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 2.20E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute Curated by ChEMBL | Assay Description In vitro inhibition of Inosine-5'-monophosphate dehydrogenase 2. | Bioorg Med Chem Lett 12: 2931-4 (2002) BindingDB Entry DOI: 10.7270/Q2SB454K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Inosine-5'-monophosphate dehydrogenase 2 (Homo sapiens (Human)) | BDBM50119116 (CHEMBL318413 | N-(3-Methoxy-4-oxazol-5-yl-phenyl)-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | >3.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute Curated by ChEMBL | Assay Description In vitro inhibition of Inosine-5'-monophosphate dehydrogenase 2. | Bioorg Med Chem Lett 12: 2931-4 (2002) BindingDB Entry DOI: 10.7270/Q2SB454K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Inosine-5'-monophosphate dehydrogenase 2 (Homo sapiens (Human)) | BDBM50119117 (CHEMBL98215 | N-(3-Methoxy-4-oxazol-5-yl-phenyl)-N...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | >3.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute Curated by ChEMBL | Assay Description The compound was tested for it's in vitro inhibitor potency against inosine monophosphate dehydrogenase II (IMPDH II) | Bioorg Med Chem Lett 12: 2931-4 (2002) BindingDB Entry DOI: 10.7270/Q2SB454K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||