

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| cGMP-specific 3',5'-cyclic phosphodiesterase (Homo sapiens (Human)) | BDBM14390 (5-[2-ethoxy-5-(4-methyl-1-piperazinylsulfonyl)phen...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | MMDB PDB PubMed | 1.90 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson & Johnson Pharmaceutical Research & Development L.L.C. Curated by ChEMBL | Assay Description Inhibitory activity against PDE5 from human corpus cavernosum | Bioorg Med Chem Lett 13: 761-5 (2003) BindingDB Entry DOI: 10.7270/Q29886CM | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| cGMP-specific 3',5'-cyclic phosphodiesterase (Homo sapiens (Human)) | BDBM50125136 (2-[2,3']Bipyridinyl-6'-yl-1-(2,3-dihydro-benzofura...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson & Johnson Pharmaceutical Research & Development L.L.C. Curated by ChEMBL | Assay Description Inhibitory activity against PDE5 from human corpus cavernosum | Bioorg Med Chem Lett 13: 761-5 (2003) BindingDB Entry DOI: 10.7270/Q29886CM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| cGMP-specific 3',5'-cyclic phosphodiesterase (Homo sapiens (Human)) | BDBM50125124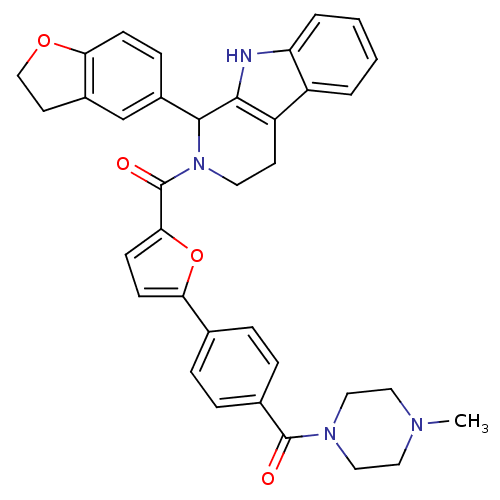 (CHEMBL433708 | [1-(2,3-Dihydro-benzofuran-5-yl)-1,...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 8.10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson & Johnson Pharmaceutical Research & Development L.L.C. Curated by ChEMBL | Assay Description Inhibitory activity against PDE5 from human corpus cavernosum | Bioorg Med Chem Lett 13: 761-5 (2003) BindingDB Entry DOI: 10.7270/Q29886CM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| cGMP-specific 3',5'-cyclic phosphodiesterase (Homo sapiens (Human)) | BDBM50118253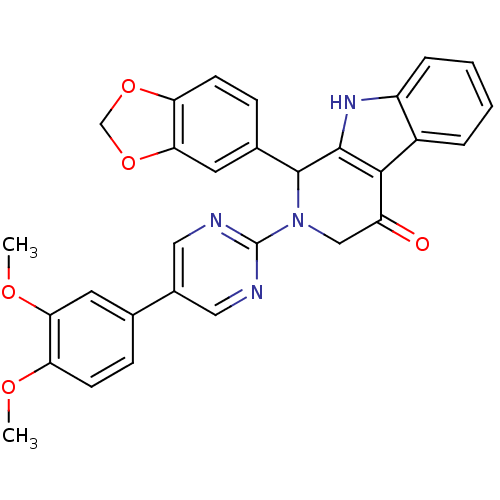 (1-Benzo[1,3]dioxol-5-yl-2-[5-(3,4-dimethoxy-phenyl...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 8.90 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson & Johnson Pharmaceutical Research & Development L.L.C. Curated by ChEMBL | Assay Description Inhibitory activity against PDE5 from human corpus cavernosum | Bioorg Med Chem Lett 13: 761-5 (2003) BindingDB Entry DOI: 10.7270/Q29886CM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| cGMP-specific 3',5'-cyclic phosphodiesterase (Homo sapiens (Human)) | BDBM50125116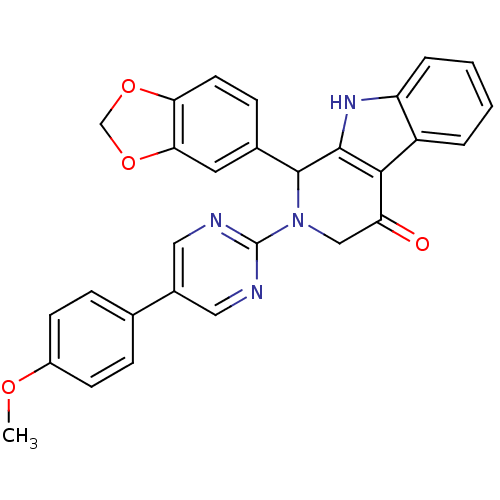 (1-Benzo[1,3]dioxol-5-yl-2-[5-(4-methoxy-phenyl)-py...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 18 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson & Johnson Pharmaceutical Research & Development L.L.C. Curated by ChEMBL | Assay Description Inhibitory activity against PDE5 from human corpus cavernosum | Bioorg Med Chem Lett 13: 761-5 (2003) BindingDB Entry DOI: 10.7270/Q29886CM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| cGMP-specific 3',5'-cyclic phosphodiesterase (Homo sapiens (Human)) | BDBM50125139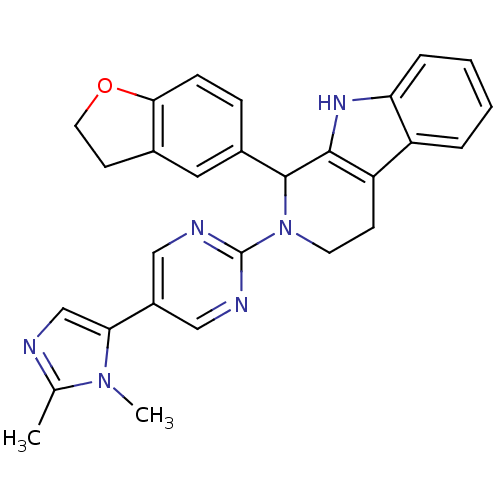 (1-(2,3-Dihydro-benzofuran-5-yl)-2-[5-(2,3-dimethyl...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 24 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson & Johnson Pharmaceutical Research & Development L.L.C. Curated by ChEMBL | Assay Description Inhibitory activity against PDE5 from human corpus cavernosum | Bioorg Med Chem Lett 13: 761-5 (2003) BindingDB Entry DOI: 10.7270/Q29886CM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| cGMP-specific 3',5'-cyclic phosphodiesterase (Homo sapiens (Human)) | BDBM50125121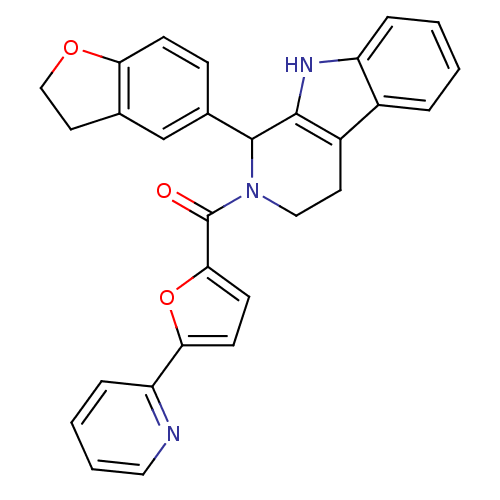 (CHEMBL349359 | [1-(2,3-Dihydro-benzofuran-5-yl)-1,...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 32 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson & Johnson Pharmaceutical Research & Development L.L.C. Curated by ChEMBL | Assay Description Inhibitory activity against PDE5 from human corpus cavernosum | Bioorg Med Chem Lett 13: 761-5 (2003) BindingDB Entry DOI: 10.7270/Q29886CM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| cGMP-specific 3',5'-cyclic phosphodiesterase (Homo sapiens (Human)) | BDBM50125143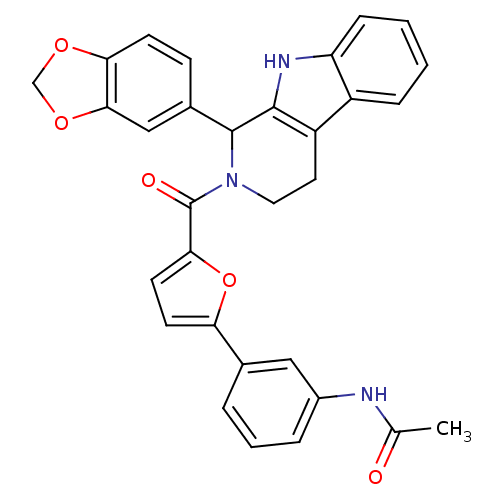 (CHEMBL166018 | N-{3-[5-(1-Benzo[1,3]dioxol-5-yl-1,...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 51 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson & Johnson Pharmaceutical Research & Development L.L.C. Curated by ChEMBL | Assay Description Inhibitory activity against PDE5 from human corpus cavernosum | Bioorg Med Chem Lett 13: 761-5 (2003) BindingDB Entry DOI: 10.7270/Q29886CM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| cGMP-specific 3',5'-cyclic phosphodiesterase (Homo sapiens (Human)) | BDBM50125118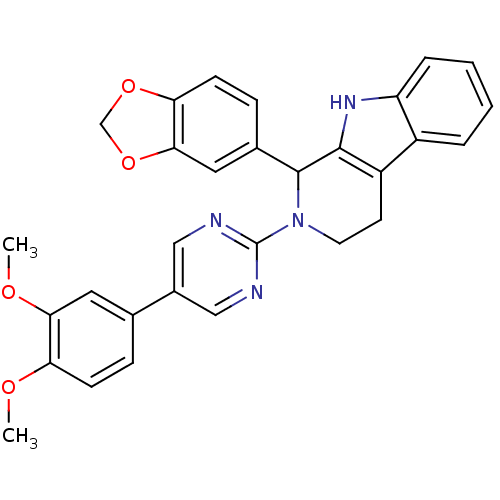 (1-Benzo[1,3]dioxol-5-yl-2-[5-(3,4-dimethoxy-phenyl...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 63 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson & Johnson Pharmaceutical Research & Development L.L.C. Curated by ChEMBL | Assay Description Inhibitory activity against PDE5 from human corpus cavernosum | Bioorg Med Chem Lett 13: 761-5 (2003) BindingDB Entry DOI: 10.7270/Q29886CM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| cGMP-specific 3',5'-cyclic phosphodiesterase (Homo sapiens (Human)) | BDBM50125118 (1-Benzo[1,3]dioxol-5-yl-2-[5-(3,4-dimethoxy-phenyl...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 63 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson & Johnson Pharmaceutical Research & Development L.L.C. Curated by ChEMBL | Assay Description Inhibitory activity against PDE5 from human corpus cavernosum | Bioorg Med Chem Lett 13: 761-5 (2003) BindingDB Entry DOI: 10.7270/Q29886CM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| cGMP-specific 3',5'-cyclic phosphodiesterase (Homo sapiens (Human)) | BDBM50125142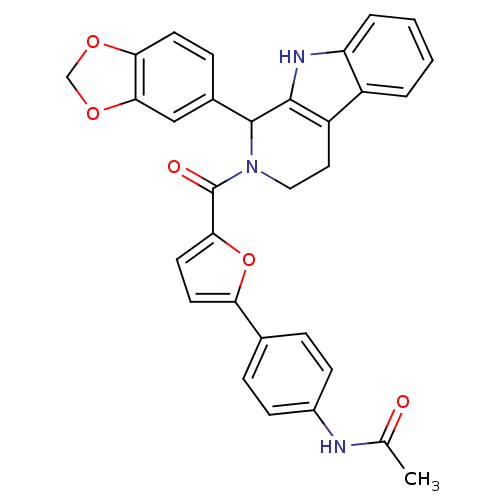 (CHEMBL349204 | N-{4-[5-(1-Benzo[1,3]dioxol-5-yl-1,...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 64 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson & Johnson Pharmaceutical Research & Development L.L.C. Curated by ChEMBL | Assay Description Inhibitory activity against PDE5 from human corpus cavernosum | Bioorg Med Chem Lett 13: 761-5 (2003) BindingDB Entry DOI: 10.7270/Q29886CM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| cGMP-specific 3',5'-cyclic phosphodiesterase (Homo sapiens (Human)) | BDBM50125141 ((1-Benzo[1,3]dioxol-5-yl-1,3,4,9-tetrahydro-beta-c...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 78 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson & Johnson Pharmaceutical Research & Development L.L.C. Curated by ChEMBL | Assay Description Inhibitory activity against PDE5 from human corpus cavernosum | Bioorg Med Chem Lett 13: 761-5 (2003) BindingDB Entry DOI: 10.7270/Q29886CM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| cGMP-specific 3',5'-cyclic phosphodiesterase (Homo sapiens (Human)) | BDBM50125135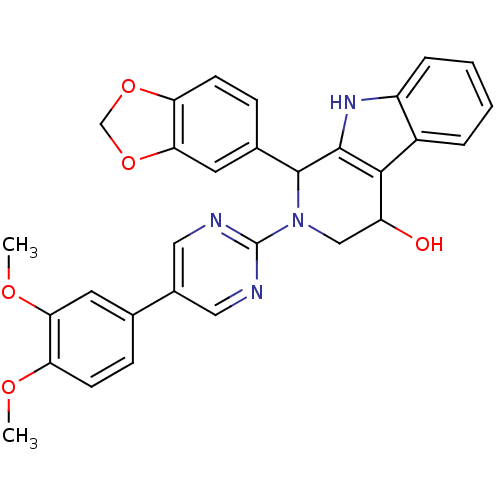 (1-Benzo[1,3]dioxol-5-yl-2-[5-(3,4-dimethoxy-phenyl...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | >100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson & Johnson Pharmaceutical Research & Development L.L.C. Curated by ChEMBL | Assay Description Inhibitory activity against PDE5 from human corpus cavernosum | Bioorg Med Chem Lett 13: 761-5 (2003) BindingDB Entry DOI: 10.7270/Q29886CM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| cGMP-specific 3',5'-cyclic phosphodiesterase (Homo sapiens (Human)) | BDBM50125128 (CHEMBL165117 | [5-(4-Amino-phenyl)-furan-2-yl]-(1-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 123 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson & Johnson Pharmaceutical Research & Development L.L.C. Curated by ChEMBL | Assay Description Inhibitory activity against PDE5 from human corpus cavernosum | Bioorg Med Chem Lett 13: 761-5 (2003) BindingDB Entry DOI: 10.7270/Q29886CM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| cGMP-specific 3',5'-cyclic phosphodiesterase (Homo sapiens (Human)) | BDBM50125144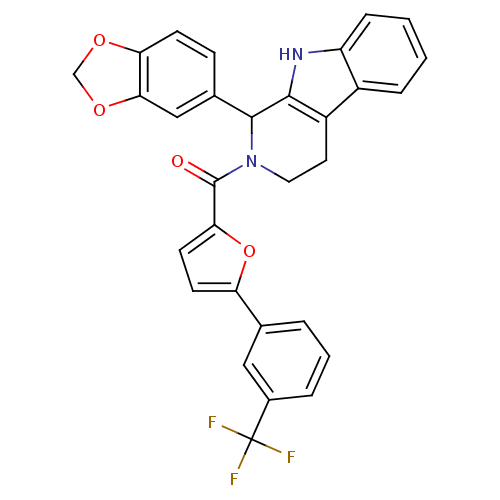 ((1-Benzo[1,3]dioxol-5-yl-1,3,4,9-tetrahydro-beta-c...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 126 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson & Johnson Pharmaceutical Research & Development L.L.C. Curated by ChEMBL | Assay Description Inhibitory activity against PDE5 from human corpus cavernosum | Bioorg Med Chem Lett 13: 761-5 (2003) BindingDB Entry DOI: 10.7270/Q29886CM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| cGMP-specific 3',5'-cyclic phosphodiesterase (Homo sapiens (Human)) | BDBM50125140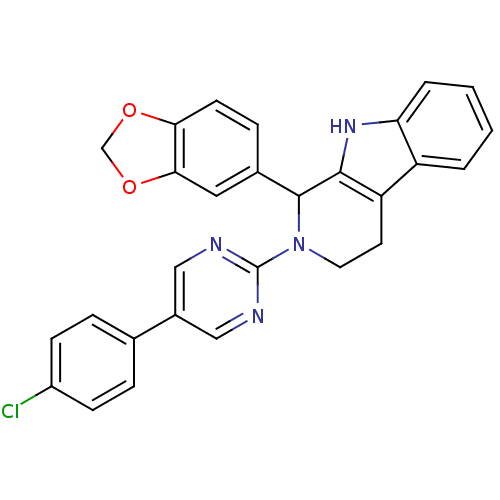 (1-Benzo[1,3]dioxol-5-yl-2-[5-(4-chloro-phenyl)-pyr...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 126 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson & Johnson Pharmaceutical Research & Development L.L.C. Curated by ChEMBL | Assay Description Inhibitory activity against PDE5 from human corpus cavernosum | Bioorg Med Chem Lett 13: 761-5 (2003) BindingDB Entry DOI: 10.7270/Q29886CM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| cGMP-specific 3',5'-cyclic phosphodiesterase (Homo sapiens (Human)) | BDBM50125129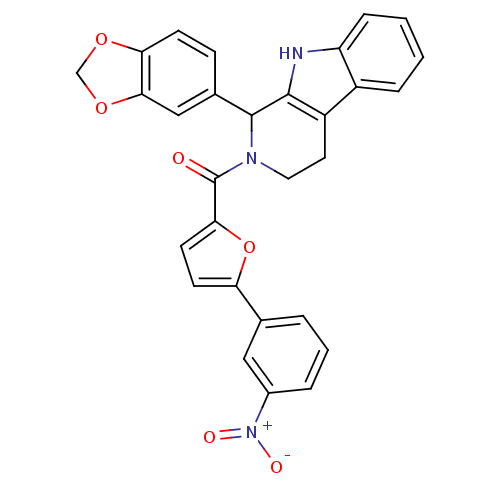 ((1-Benzo[1,3]dioxol-5-yl-1,3,4,9-tetrahydro-beta-c...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 130 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson & Johnson Pharmaceutical Research & Development L.L.C. Curated by ChEMBL | Assay Description Inhibitory activity against PDE5 from human corpus cavernosum | Bioorg Med Chem Lett 13: 761-5 (2003) BindingDB Entry DOI: 10.7270/Q29886CM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| cGMP-specific 3',5'-cyclic phosphodiesterase (Homo sapiens (Human)) | BDBM50125122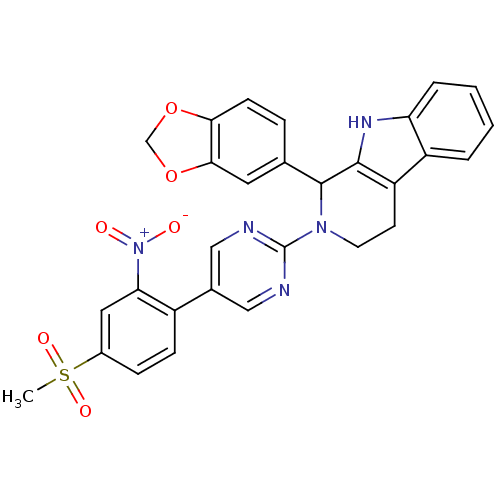 (1-Benzo[1,3]dioxol-5-yl-2-[5-(4-methanesulfonyl-2-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 132 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson & Johnson Pharmaceutical Research & Development L.L.C. Curated by ChEMBL | Assay Description Inhibitory activity against PDE5 from human corpus cavernosum | Bioorg Med Chem Lett 13: 761-5 (2003) BindingDB Entry DOI: 10.7270/Q29886CM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| cGMP-specific 3',5'-cyclic phosphodiesterase (Homo sapiens (Human)) | BDBM50125127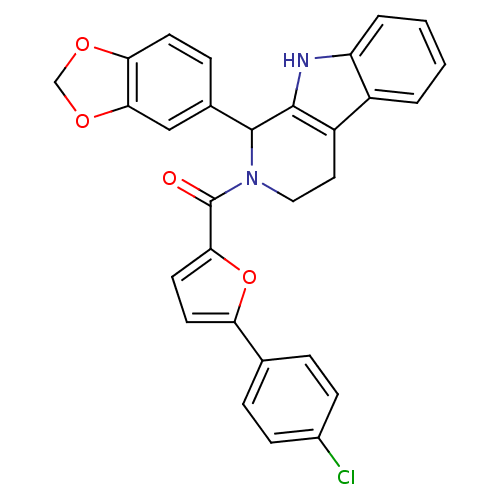 ((1-Benzo[1,3]dioxol-5-yl-1,3,4,9-tetrahydro-beta-c...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 145 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson & Johnson Pharmaceutical Research & Development L.L.C. Curated by ChEMBL | Assay Description Inhibitory activity against PDE5 from human corpus cavernosum | Bioorg Med Chem Lett 13: 761-5 (2003) BindingDB Entry DOI: 10.7270/Q29886CM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| cGMP-specific 3',5'-cyclic phosphodiesterase (Homo sapiens (Human)) | BDBM50125123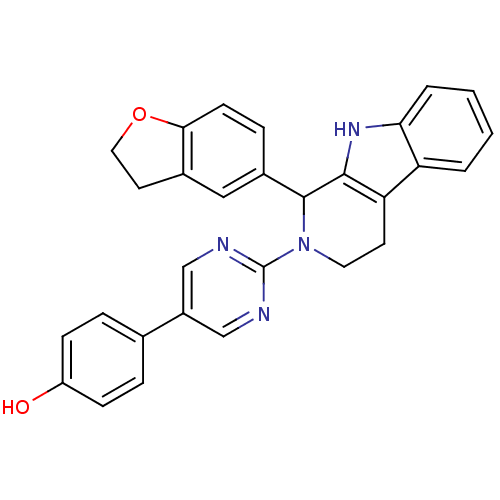 (4-{2-[1-(2,3-Dihydro-benzofuran-5-yl)-1,3,4,9-tetr...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 154 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson & Johnson Pharmaceutical Research & Development L.L.C. Curated by ChEMBL | Assay Description Inhibitory activity against PDE5 from human corpus cavernosum | Bioorg Med Chem Lett 13: 761-5 (2003) BindingDB Entry DOI: 10.7270/Q29886CM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| cGMP-specific 3',5'-cyclic phosphodiesterase (Homo sapiens (Human)) | BDBM50125131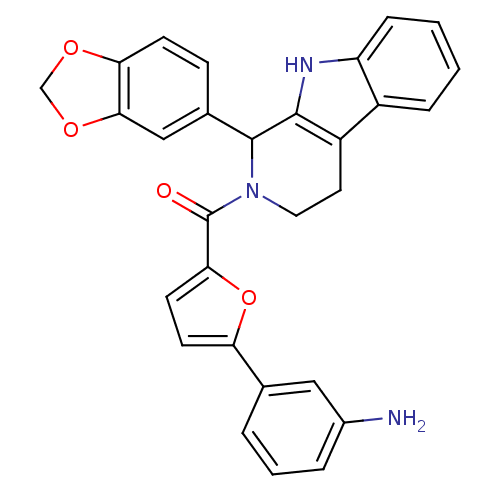 (CHEMBL350962 | [5-(3-Amino-phenyl)-furan-2-yl]-(1-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 179 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson & Johnson Pharmaceutical Research & Development L.L.C. Curated by ChEMBL | Assay Description Inhibitory activity against PDE5 from human corpus cavernosum | Bioorg Med Chem Lett 13: 761-5 (2003) BindingDB Entry DOI: 10.7270/Q29886CM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| cGMP-specific 3',5'-cyclic phosphodiesterase (Homo sapiens (Human)) | BDBM50125134 (1-(2,3-Dihydro-benzofuran-5-yl)-2-[5-(4-methoxy-ph...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 188 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson & Johnson Pharmaceutical Research & Development L.L.C. Curated by ChEMBL | Assay Description Inhibitory activity against PDE5 from human corpus cavernosum | Bioorg Med Chem Lett 13: 761-5 (2003) BindingDB Entry DOI: 10.7270/Q29886CM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| cGMP-specific 3',5'-cyclic phosphodiesterase (Homo sapiens (Human)) | BDBM50125134 (1-(2,3-Dihydro-benzofuran-5-yl)-2-[5-(4-methoxy-ph...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 189 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson & Johnson Pharmaceutical Research & Development L.L.C. Curated by ChEMBL | Assay Description Inhibitory activity against PDE5 from human corpus cavernosum | Bioorg Med Chem Lett 13: 761-5 (2003) BindingDB Entry DOI: 10.7270/Q29886CM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| cGMP-specific 3',5'-cyclic phosphodiesterase (Homo sapiens (Human)) | BDBM50125137 ((1-Benzo[1,3]dioxol-5-yl-1,3,4,9-tetrahydro-beta-c...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 219 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson & Johnson Pharmaceutical Research & Development L.L.C. Curated by ChEMBL | Assay Description Inhibitory activity against PDE5 from human corpus cavernosum | Bioorg Med Chem Lett 13: 761-5 (2003) BindingDB Entry DOI: 10.7270/Q29886CM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| cGMP-specific 3',5'-cyclic phosphodiesterase (Homo sapiens (Human)) | BDBM50125130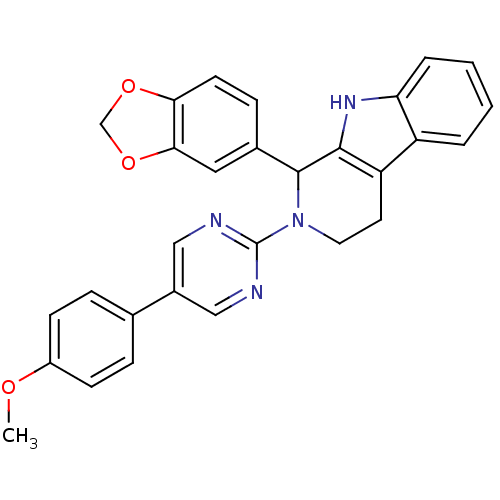 (1-Benzo[1,3]dioxol-5-yl-2-[5-(4-methoxy-phenyl)-py...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 246 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson & Johnson Pharmaceutical Research & Development L.L.C. Curated by ChEMBL | Assay Description Inhibitory activity against PDE5 from human corpus cavernosum | Bioorg Med Chem Lett 13: 761-5 (2003) BindingDB Entry DOI: 10.7270/Q29886CM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| cGMP-specific 3',5'-cyclic phosphodiesterase (Homo sapiens (Human)) | BDBM50125130 (1-Benzo[1,3]dioxol-5-yl-2-[5-(4-methoxy-phenyl)-py...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 246 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson & Johnson Pharmaceutical Research & Development L.L.C. Curated by ChEMBL | Assay Description Inhibitory activity against PDE5 from human corpus cavernosum | Bioorg Med Chem Lett 13: 761-5 (2003) BindingDB Entry DOI: 10.7270/Q29886CM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| cGMP-specific 3',5'-cyclic phosphodiesterase (Homo sapiens (Human)) | BDBM50125133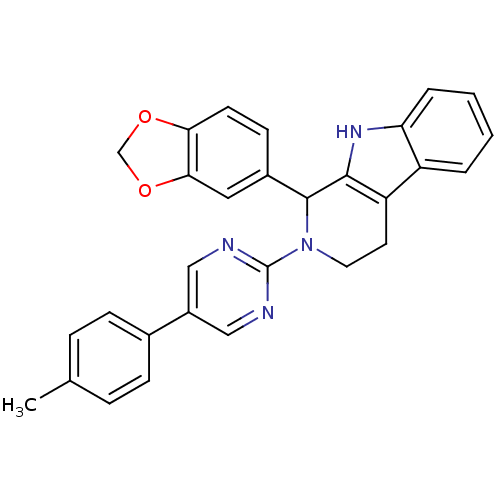 (1-Benzo[1,3]dioxol-5-yl-2-(5-p-tolyl-pyrimidin-2-y...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 543 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson & Johnson Pharmaceutical Research & Development L.L.C. Curated by ChEMBL | Assay Description Inhibitory activity against PDE5 from human corpus cavernosum | Bioorg Med Chem Lett 13: 761-5 (2003) BindingDB Entry DOI: 10.7270/Q29886CM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| cGMP-specific 3',5'-cyclic phosphodiesterase (Homo sapiens (Human)) | BDBM50125126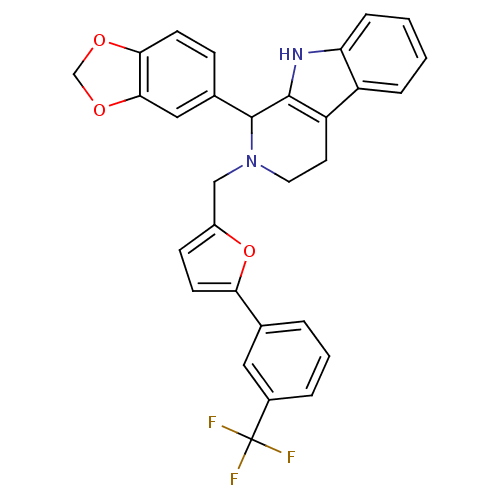 (1-Benzo[1,3]dioxol-5-yl-2-[5-(3-trifluoromethyl-ph...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 850 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson & Johnson Pharmaceutical Research & Development L.L.C. Curated by ChEMBL | Assay Description Inhibitory activity against PDE5 from human corpus cavernosum | Bioorg Med Chem Lett 13: 761-5 (2003) BindingDB Entry DOI: 10.7270/Q29886CM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| cGMP-specific 3',5'-cyclic phosphodiesterase (Homo sapiens (Human)) | BDBM50125120 (1-(2,3-Dihydro-benzofuran-5-yl)-2-{5-[4-(2-pyrroli...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | >1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson & Johnson Pharmaceutical Research & Development L.L.C. Curated by ChEMBL | Assay Description Inhibitory activity against PDE5 from human corpus cavernosum | Bioorg Med Chem Lett 13: 761-5 (2003) BindingDB Entry DOI: 10.7270/Q29886CM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| cGMP-specific 3',5'-cyclic phosphodiesterase (Homo sapiens (Human)) | BDBM50125117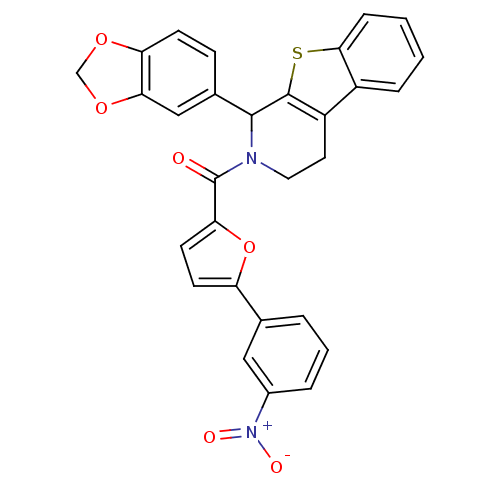 ((1-Benzo[1,3]dioxol-5-yl-3,4-dihydro-1H-benzo[4,5]...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | PubMed | >5.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson & Johnson Pharmaceutical Research & Development L.L.C. Curated by ChEMBL | Assay Description Inhibitory activity against PDE5 from human corpus cavernosum | Bioorg Med Chem Lett 13: 761-5 (2003) BindingDB Entry DOI: 10.7270/Q29886CM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| cGMP-specific 3',5'-cyclic phosphodiesterase (Homo sapiens (Human)) | BDBM50125119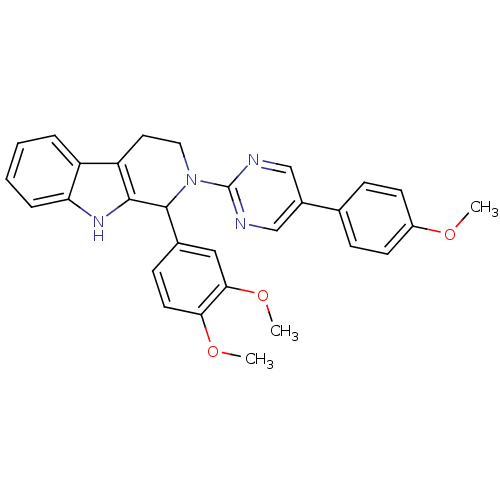 (1-(3,4-Dimethoxy-phenyl)-2-[5-(4-methoxy-phenyl)-p...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson & Johnson Pharmaceutical Research & Development L.L.C. Curated by ChEMBL | Assay Description Inhibitory activity against PDE5 from human corpus cavernosum | Bioorg Med Chem Lett 13: 761-5 (2003) BindingDB Entry DOI: 10.7270/Q29886CM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| cGMP-specific 3',5'-cyclic phosphodiesterase (Homo sapiens (Human)) | BDBM50125132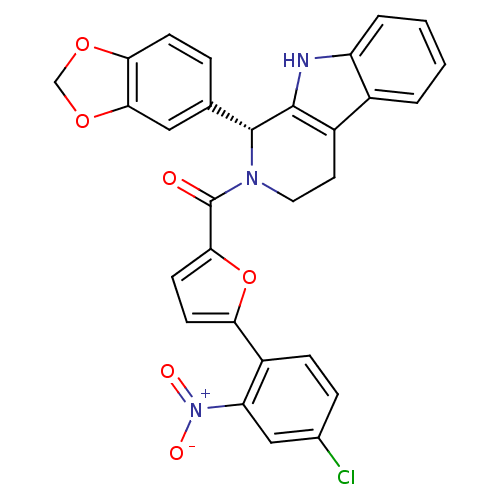 (((R)-1-Benzo[1,3]dioxol-5-yl-1,3,4,9-tetrahydro-be...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 67 | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson & Johnson Pharmaceutical Research & Development L.L.C. Curated by ChEMBL | Assay Description Inhibition of PDE5 (Phosphodiesterase) from human corpus cavernosum | Bioorg Med Chem Lett 13: 761-5 (2003) BindingDB Entry DOI: 10.7270/Q29886CM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| cGMP-specific 3',5'-cyclic phosphodiesterase (Homo sapiens (Human)) | BDBM50125146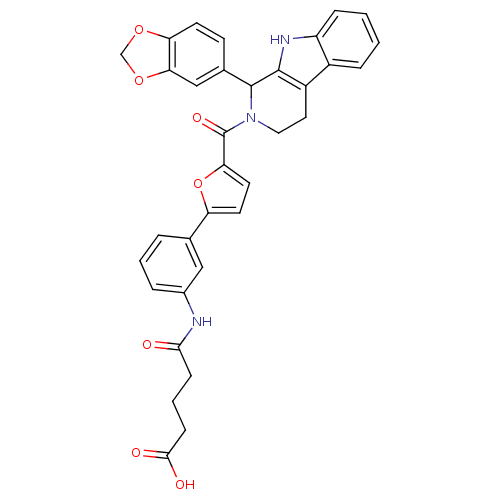 (4-{3-[5-(1-Benzo[1,3]dioxol-5-yl-1,3,4,9-tetrahydr...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 95 | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson & Johnson Pharmaceutical Research & Development L.L.C. Curated by ChEMBL | Assay Description Inhibition of PDE5 (Phosphodiesterase) from human corpus cavernosum | Bioorg Med Chem Lett 13: 761-5 (2003) BindingDB Entry DOI: 10.7270/Q29886CM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| cGMP-specific 3',5'-cyclic phosphodiesterase (Homo sapiens (Human)) | BDBM50125147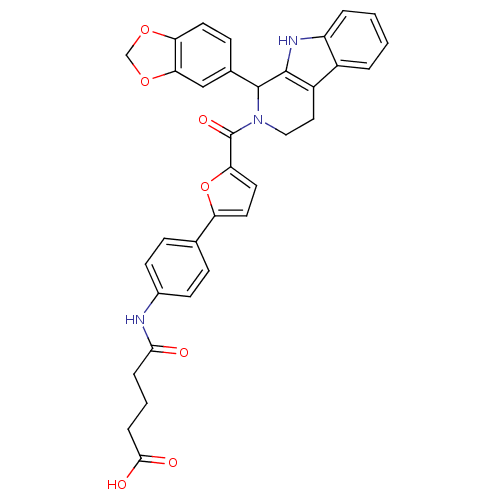 (4-{4-[5-(1-Benzo[1,3]dioxol-5-yl-1,3,4,9-tetrahydr...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 100 | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson & Johnson Pharmaceutical Research & Development L.L.C. Curated by ChEMBL | Assay Description Inhibition of PDE5 (Phosphodiesterase) from human corpus cavernosum | Bioorg Med Chem Lett 13: 761-5 (2003) BindingDB Entry DOI: 10.7270/Q29886CM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| cGMP-specific 3',5'-cyclic phosphodiesterase (Homo sapiens (Human)) | BDBM50125138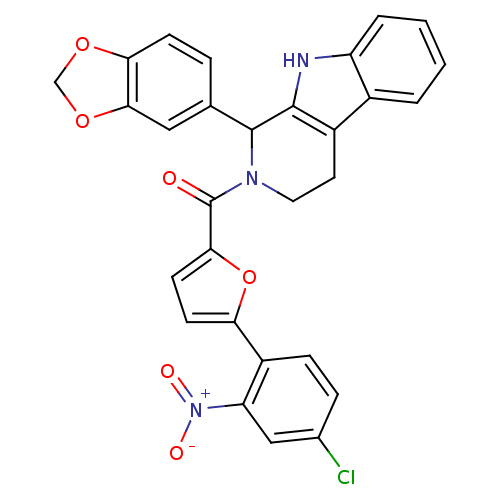 ((1-Benzo[1,3]dioxol-5-yl-1,3,4,9-tetrahydro-beta-c...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 105 | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson & Johnson Pharmaceutical Research & Development L.L.C. Curated by ChEMBL | Assay Description Inhibition of PDE5 (Phosphodiesterase) from human corpus cavernosum | Bioorg Med Chem Lett 13: 761-5 (2003) BindingDB Entry DOI: 10.7270/Q29886CM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| cGMP-specific 3',5'-cyclic phosphodiesterase (Homo sapiens (Human)) | BDBM50125145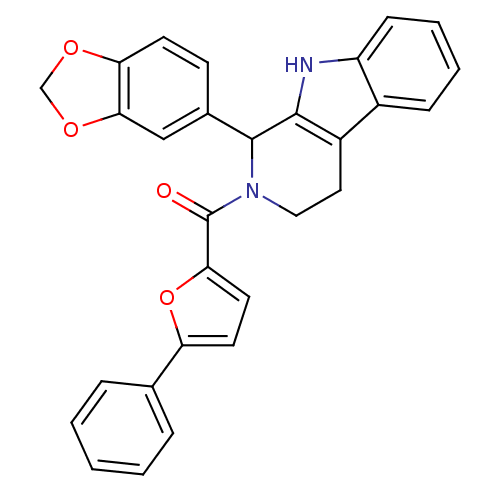 ((1-Benzo[1,3]dioxol-5-yl-1,3,4,9-tetrahydro-beta-c...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 750 | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson & Johnson Pharmaceutical Research & Development L.L.C. Curated by ChEMBL | Assay Description Inhibition of PDE5 (Phosphodiesterase) from human corpus cavernosum | Bioorg Med Chem Lett 13: 761-5 (2003) BindingDB Entry DOI: 10.7270/Q29886CM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| cGMP-specific 3',5'-cyclic phosphodiesterase (Homo sapiens (Human)) | BDBM50125125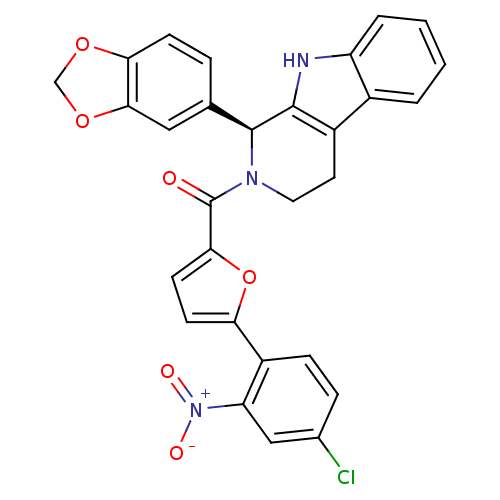 (((S)-1-Benzo[1,3]dioxol-5-yl-1,3,4,9-tetrahydro-be...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson & Johnson Pharmaceutical Research & Development L.L.C. Curated by ChEMBL | Assay Description Inhibition of PDE5 (Phosphodiesterase) from human corpus cavernosum | Bioorg Med Chem Lett 13: 761-5 (2003) BindingDB Entry DOI: 10.7270/Q29886CM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||