

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Muscarinic acetylcholine receptor M2 (Homo sapiens (Human)) | BDBM50411207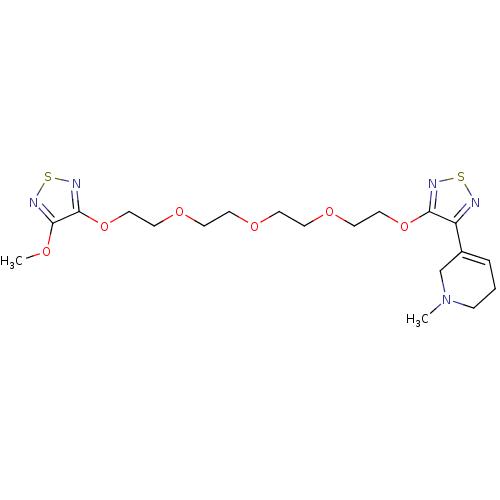 (CHEMBL216927 | CHEMBL553058) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.5 | n/a | n/a | n/a | n/a | n/a | n/a |
The University of Toledo Curated by ChEMBL | Assay Description Inhibitory activity was evaluated against Muscarinic acetylcholine receptor M2 expressed in A9 L cells | J Med Chem 46: 4273-86 (2003) Article DOI: 10.1021/jm0301235 BindingDB Entry DOI: 10.7270/Q2MG7QT4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Muscarinic acetylcholine receptor M2 (Homo sapiens (Human)) | BDBM50107697 (1-[4-(1-methyl-1,2,3,6-tetrahydro-5-pyridinyl)-1,2...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 0.630 | n/a | n/a | n/a | n/a | n/a | n/a |
The University of Toledo Curated by ChEMBL | Assay Description Inhibitory activity was evaluated against Muscarinic acetylcholine receptor M2 expressed in A9 L cells | J Med Chem 46: 4273-86 (2003) Article DOI: 10.1021/jm0301235 BindingDB Entry DOI: 10.7270/Q2MG7QT4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Muscarinic acetylcholine receptor M2 (Homo sapiens (Human)) | BDBM50004656 ((2-Carbamoyloxy-ethyl)-trimethyl-ammonium | (2-Car...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank KEGG MMDB PC cid PC sid PDB UniChem Similars | Article PubMed | n/a | n/a | 13 | n/a | n/a | n/a | n/a | n/a | n/a |
The University of Toledo Curated by ChEMBL | Assay Description Inhibitory activity was evaluated against Muscarinic acetylcholine receptor M2 expressed in A9 L cells | J Med Chem 46: 4273-86 (2003) Article DOI: 10.1021/jm0301235 BindingDB Entry DOI: 10.7270/Q2MG7QT4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Muscarinic acetylcholine receptor M2 (Homo sapiens (Human)) | BDBM50003359 (5-(4-Hexyloxy-[1,2,5]thiadiazol-3-yl)-1-methyl-1,2...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE KEGG PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | 13 | n/a | n/a | n/a | n/a | n/a | n/a |
The University of Toledo Curated by ChEMBL | Assay Description Inhibitory activity was evaluated against Muscarinic acetylcholine receptor M2 expressed in A9 L cells | J Med Chem 46: 4273-86 (2003) Article DOI: 10.1021/jm0301235 BindingDB Entry DOI: 10.7270/Q2MG7QT4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Muscarinic acetylcholine receptor M4 (Homo sapiens (Human)) | BDBM50411207 (CHEMBL216927 | CHEMBL553058) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 16 | n/a | n/a | n/a | n/a | n/a | n/a |
The University of Toledo Curated by ChEMBL | Assay Description Inhibitory activity was evaluated against Muscarinic acetylcholine receptor M4 expressed in A9 L cells ; Not determined | J Med Chem 46: 4273-86 (2003) Article DOI: 10.1021/jm0301235 BindingDB Entry DOI: 10.7270/Q2MG7QT4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Muscarinic acetylcholine receptor M4 (Homo sapiens (Human)) | BDBM50003359 (5-(4-Hexyloxy-[1,2,5]thiadiazol-3-yl)-1-methyl-1,2...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE KEGG PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | n/a | n/a | 25 | n/a | n/a | n/a | n/a | n/a | n/a |
The University of Toledo Curated by ChEMBL | Assay Description Inhibitory activity was evaluated against Muscarinic acetylcholine receptor M4 expressed in A9 L cells | J Med Chem 46: 4273-86 (2003) Article DOI: 10.1021/jm0301235 BindingDB Entry DOI: 10.7270/Q2MG7QT4 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Muscarinic acetylcholine receptor M4 (Homo sapiens (Human)) | BDBM50004656 ((2-Carbamoyloxy-ethyl)-trimethyl-ammonium | (2-Car...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank KEGG MMDB PC cid PC sid PDB UniChem Similars | Article PubMed | n/a | n/a | 1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
The University of Toledo Curated by ChEMBL | Assay Description Inhibitory activity was evaluated against Muscarinic acetylcholine receptor M4 expressed in A9 L cells | J Med Chem 46: 4273-86 (2003) Article DOI: 10.1021/jm0301235 BindingDB Entry DOI: 10.7270/Q2MG7QT4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||